-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biological Engineering
p-ISSN: 2163-1875 e-ISSN: 2163-1883
2013; 3(2): 11-17
doi:10.5923/j.ijbe.20130302.01
Optical Imaging of Motor Cortical Hemodynamic Response to Directional Arm Movements Using Near-infrared Spectroscopy
Nicoladie D Tam1, George Zouridakis2
1Department of Biological Sciences, University of North Texas, Denton, TX 76023, USA
2Departments of Engineering Technology, Computer Science, and Electrical & Computer Engineering, University of Houston, Houston, TX 77204, USA
Correspondence to: Nicoladie D Tam, Department of Biological Sciences, University of North Texas, Denton, TX 76023, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study aims at determining arm-movement directions from functional near-infrared spectroscopy (fNIRS) hemodynamic signals in order to decode intentional motor commands, originating in the motor cortices of humans, which could be implemented in neuroprosthetic assistive devices for assisting the physically disabled. Motor cortical hemodynamic responses were recorded using 64 spatially distributed optrodes from 14 normal subjects during free arm orthogonal movements in the x- and y-directions on a horizontal plane. The time course of oxy-(HbO2) and deoxy-hemoglobin (Hb), and of their summation (HbO2 + Hb) and difference (HbO2 – Hb) signals, representing the hemodynamic profiles of total oxygen delivery and extraction, respectively, were computed for the localized neuronal populations in the motor cortices underlying the optrodes. Analysis of the above hemodynamic signals revealed that they could be temporally, spatially, or spatiotemporally decoupled, depending on the movement direction. Thus, by analyzing the spatiotemporal profiles of brain activation we could identify the direction of the orthogonal movements uniquely. Our findings demonstrate that movement direction, a key feature of motor commands, can be reliably extracted in real-time from surface recorded fNIRS signals, and support their viability in future noninvasive assistive devices.
Keywords: Near-infrared Spectroscopy, Motor Cortex, Directional Arm Movements, Optical Imaging, Hemodynamic Response
Cite this paper: Nicoladie D Tam, George Zouridakis, Optical Imaging of Motor Cortical Hemodynamic Response to Directional Arm Movements Using Near-infrared Spectroscopy, International Journal of Biological Engineering, Vol. 3 No. 2, 2013, pp. 11-17. doi: 10.5923/j.ijbe.20130302.01.
Article Outline
1. Introduction
- Functional near-infrared spectroscopy (fNIRS) is a powerful noninvasive optical imaging technique to detect differential changes in hemodynamic response to oxygen delivery and extraction to the underlying neural tissues. Near-infrared (NIR) light can penetrate biological tissues up the depth of approximately 2 cm in the cortex without significant degradation of the optical signals. The depth-related information on absorption variation can be estimated by finite element simulation methods[1]. Hemoglobin molecules absorb light in the NIR spectral region and can discern the difference between oxy-hemoglobin (HbO2) and deoxy-hemoglobin (Hb) based on the absorption spectral signature produced by the molecular chemical bonds. This allows real-time monitoring of the hemodynamic signals in brain tissues in a noninvasive fashion[2-4]. The characteristic absorption spectra of HbO2 and Hb at the NIR region recorded on the scalp can be used to detect the oxygen demands of the underlying brain tissues in the cortex. This allows real-time detection of brain activation based on metabolic events (neuro-hemodynamics) related to oxygen consumption of the underlying neural tissues.fNIRS has the advantage of detecting not just HbO2 but also Hb levels in real-time. The detection and differentiation of HbO2 and Hb levels are computed from the modified Beer-Lambert law[5] based on the characteristic absorption spectra of hemoglobin molecules when incident NIR lights are shined onto the neural tissues. In neuroimaging, the detector records the refracted light scattered by the tissue rather than the transmitted light as in conventional oximeter, because both emitters and detectors reside on the same side of the scalp surface. The depth of recording is related to the distance between emitter and detector according to the ray tracing of scattered-light paths from emitter to detector. Using multichannel emitters/sensors, high-resolution hemodynamic signals can be recorded temporally (in msec) in the cortex together with spatial resolution in cm. Thus, it can localize the cortical region where neural vascular activation/deactivation occurs (as represented by the HbO2 and Hb signals) complementary to functional magnetic resonance imaging (fMRI).It is well known that brain activation is correlated with the oxygen consumption of the underlying neural tissues[2-4] and is represented by the hemodynamics of local oxygen delivery (HbO2) and oxygen extraction (Hb) at an activated site of neural tissue. The ability to detect both HbO2 and Hb will allow differentiation of the complex dynamics in oxygen delivery and extraction in response to neural activation and deactivation. Although it has been demonstrated that neuronal activation and vascular responses are correlated by the so-called “neurovascular coupling”[6], analysis of various differential measures of fNIRS signals will allow us to determine whether temporal decoupling of neurovascular responses (oxygen delivery vs. oxygen extraction) may occur in real-time during a cognitive task that involves motor activation. There is evidence from simulation models that the transient blood-oxygen-level-dependent (BOLD) signals detected in fMRI may not necessarily reflect cerebral blood flow (CBF), cerebral metabolic rate of oxygen (CMRO2), or cerebral blood volume (CBV)[7], although biophysical models showed that there is high correlation between ASL (arterial spin labeling)-based fMRI, BOLD and NIRS during an event-related motor activity in human subjects[8]. A decoupled hemodynamic response between oxygen delivery and extraction, if it exists, may shed light on the complex dynamics of neural activation and deactivation differentially. Using optical imaging analysis, neurovascular decoupling in oxygen delivery and extraction may be revealed spatially, temporally, and spatiotemporally.As a brief review, HbO2 and Hb signals are computed by the modified Beer-Lambert Law[5]:
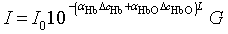 | (1) |
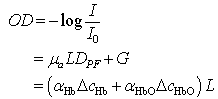 | (2) |
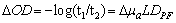 | (3) |
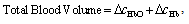 | (4) |
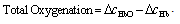 | (5) |
2. Material and Methods
- Fourteen normal subjects were recruited in this experiment. The experimental protocol was approved by the Institutional Review Board. We employed a simple movement execution experimental paradigm by asking subjects to produce volitional free arm movements on a horizontal xy-plane parallel to a desk surface. We instructed subjects to execute right-left (or front-back) hand movements between two fixed targets 30 cm apart along x-axis, and two fixed targets 30 cm apart along the y-axis, on the xy-plane, while we recorded multichannel fNIRS optical signals from both motor cortices bilaterally. We used the 64-channel ISS Imagent™ optical system to record the hemodynamic signals (HbO2 and Hb) from the subjects. Each subject was asked to perform right-left arm movements (or front-back movements in different experiments) consecutively for 5 minutes. The movements were either cued to initiate movement by a sound tone rang at 5 sec intervals or self-paced without any auditory stimulus cues. This repeated trial paradigm allows us to improve the signal-to-noise ratio in our analysis by averaging the directional movement aligned to either the onset of the sound tone or onset of movement (i.e., right, left, front and back movements were averaged separately for each subject in our analysis). The results presented in this paper included trials that are cued on the sound tone so as to include both phases of movement preparation (which is known to be involving the premotor cortical activation) and movement direction command (which is known to be involving the motor cortical activation) in our analysis.
3. Results
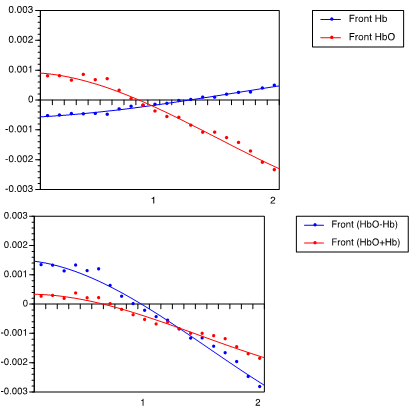
- Figs. 1-4 showed the hemodynamic signals representing HbO2 and Hb levels during right-left and front-back arm movements recorded from the motor cortex. The hemodynamic graphs revealed distinct changes in HbO2 and Hb signals that were correlated with the orthogonal movement direction, distinguishing between right-left vs. front-back arm movements.Fig. 1 showed the hemodynamic signals for arm movements in the front direction (+y direction). Fig. 1A shows changes in HbO2 (red) and Hb (blue). The temporal changes during frontward arm movement revealed a continuous decrease of oxygen delivery, and a slight increase in oxygen extraction. During this arm movement, oxygen delivery and extraction were decoupled, with temporally diverging responses. That is, oxygen delivery and extraction changed in opposite directions.
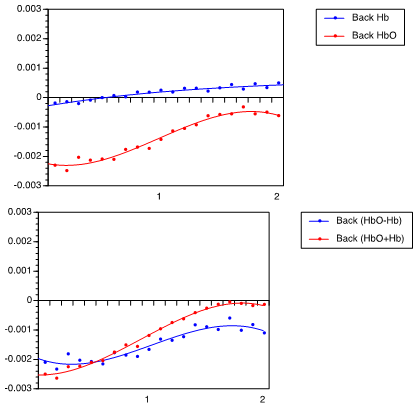 | Figure 2. Hemodynamic response of 30-cm arm movement toward back direction, (A) showing HbO2 and Hb levels, and (B) showing (HbO2 + Hb) and (HbO2 – Hb) |
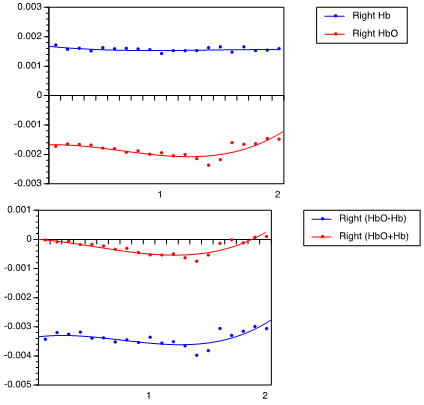 | Figure 3. Hemodynamic response of rightward arm movement, (A) showing HbO2 and Hb levels, and (B) showing (HbO2 + Hb) and (HbO2 – Hb) |
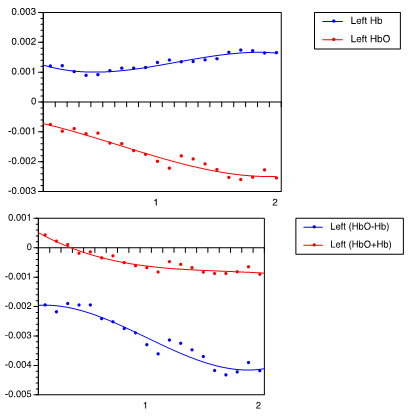 | Figure 4. Hemodynamic response of leftward arm movement, (A) showing HbO2 and Hb levels, and (B) showing (HbO2 + Hb) and (HbO2 – Hb) |
4. Discussions
- The analysis of optical signals representing the hemodynamic responses demonstrates that directional movements could be decoded from multiple measures of hemodynamic responses. Specifically, different localized motor cortical areas are involved in specific task-related directional movement control. This illustrates that the +x, –x, +y, –y directions can be differentially decoded from different optrodes of fNIRS recordings. These orthogonal directional controls can then be used to drive a wheelchair in future neuroprosthetic design to assist the physically disabled. These results show that intentional movement direction can be detected from the optical signals monitored noninvasively from motor cortex. This demonstrates the feasibility of decoding the intentional movement directions (right-left and front-back) from optical signals. That is, the intentional directions of +x vs. –x and +y vs. –y can be detected based on the fNIRS signals, representing distinct localized neural populations participating in the different population vector encoding for movement directions. The differential activation and deactivation of hemodynamic responses can be used to map specific movement intentions temporally.Most interestingly, the analysis reveals that different measures of hemodynamic vascular responses exhibited decoupled responses dynamically during task-related movement control. Oxygen delivery and extraction can be coupled temporally in one movement direction, but partially decoupled in opposite movement direction. The decoupling can occur in opposite manner (i.e., activation vs. deactivation), or it can decouple by not responding (i.e., remain constant throughout the task-event). When the hemodynamic measures corresponding to the vascular events are decoupled temporally during a task, it is unknown how such hemodynamic signals are coupled/decoupled with respect to the electrical firing activities of the neurons. It remains to be determined how hemodynamic vascular response is related temporally to the neuron firing activities or synaptic events. The hemodynamic response is more likely related to the metabolic events, which may involve not only electrical firing of neurons and synaptic activation, but also metabolic activities of supportive cells, such as glial cells in regulating ionic concentration and neurotransmitter metabolism.Furthermore, the differential changes in the temporal profile of hemodynamic signals reveals that oxygen demands can be accomplished by two independent processes: oxygen extraction and oxygen delivery. Total oxygen delivery is maximized by increasing total blood volume delivered (HbO2 + Hb) and by increasing partial pressure of oxygen (PO2) saturation. Oxygen extraction can be accomplished by unloading oxygen molecules from hemoglobin molecules, as detected by a decrease in PO2 in the hemodynamic signal. This phenomenon is often observed independently during physical exercise in the periphery (such as muscles, which occurs outside the central nervous system), when oxygen extraction exceeded the ability for oxygen to delivery to the target tissue by hemoglobin molecules. When oxygen demand is not as intense, oxygen delivery is often sufficiently met by the total blood volume delivered by the vessels under normal circumstances, with PO2 oxygen maintained at 96–99% saturation normally. It is only during hypoxic condition that PO2 saturation in hemoglobin molecules drops below 96%.It can be inferred from this hemodynamic phenomenon that neural metabolic demands may increase momentarily such that micro-hypoxic condition occurs locally at the activation site. If normal (non-hypoxic) condition occurs without exceeding this limit, the oxygen extraction signal may remain constant, which can account for the above differential temporal decoupled phenomenon in the experiment illustrated earlier. This is consistent with the complex transient biphasic temporal cerebral oxygenation responses reported in response to intense oscillatory motor stimulation[22]. Similar changes in regional cerebral blood oxygenation (rCBO) over the motor cortex and blood flow velocity changes (CBFV) in the middle cerebral artery were reported in a combined NIRS and transcranial Doppler Sonography (TCD) study for sequential finger opposition task[24]. Interestingly, it has been reported by[23] that the size of hemodynamic response is dependent on the resting period between sequential motor execution, with maximal response peaked at 30 sec resting period in finger tapping task. This may suggest micro-hypoxic condition might have occurred when oxygen demand exceeds oxygen delivery when the movement is executed too rapidly.Thus, these findings are consistent with the evidence that the hemodynamic responses are complex dynamical interactions among many different vascular components to produce the differential changes spatially, temporally and spatiotemporally. The results show that oxygen delivery and extraction can change independently and decoupled temporally, such that if only one of these hemodynamic responses (either HbO2 or Hb) is used to characterize the neuronal activation/deactivation response without taking the other response into account, it will miss the complete description of the overall response that is representative of the oxygen delivery and extraction dynamics.
5. Conclusions
- This study revealed three major findings in using multiple hemodynamic measures to decode intentional directional arm movements. First, orthogonal movement directions can be differentiated based on the differential temporal changes in hemodynamic measures of HbO2, Hb, (HbO2 + Hb) and (HbO2 – Hb) NIRS signals. Second, the increase/decrease in the above hemodynamic measures can be decoupled from each other temporally during the directional motor task. Third, orthogonal movement directions can also be differentiated by different hemodynamic activation / deactivation patterns from different localized population of neurons, each representing different population vector responses.Our findings suggest that fNIRS-based brain activity analysis to decode intentional movement direction provides a viable alternative to most current prosthetic devices that require invasive microelectrode brain implants.
ACKNOWLEDGEMENTS
- We thank Ms. Krista Smith for the helpful suggestions, and for proofreading the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML