-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biological Engineering
p-ISSN: 2163-1875 e-ISSN: 2163-1883
2012; 2(4): 27-38
doi: 10.5923/j.ijbe.20120204.01
RiceWrist Robotic Device for Upper Limb Training: Feasibility Study and Case Report of Two Tetraplegic Persons with Spinal Cord Injury
Z. Kadivar 1, J. L. Sullivan 2, D. P. Eng 2, A. U. Pehlivan 2, M. K. O. Malley 2, N. Yozbatiran 3, J. D. O. Berliner 3, C. Boake 3, G. E. Francisco 3
1Department of Physical Medicine and Rehabilitation Baylor College of Medicine, Houston, TX, U.S.A
2Department of Mechanical Engineering and Material Science Rice University, Houston, U.S.A
3Department of Physical Medicine and Rehabilitation, University of Texas Medical School, Houston, U.S.A
Correspondence to: Z. Kadivar , Department of Physical Medicine and Rehabilitation Baylor College of Medicine, Houston, TX, U.S.A.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Regaining upper extremity function is the primary concern of persons with tetraplegia caused by spinal cord injury (SCI). Robotic rehabilitation has been inadequately tested and underutilized in rehabilitation of the upper extremity in the SCI population. Given the acceptance of robotic training in stroke rehabilitation and SCI gait training, coupled with recent evidence that the spinal cord, like the brain, demonstrates plasticity that can be enhanced by repetitive movement training such as that available with robotic devices, it is probable that robotic upper extremity training of persons with SCI could be clinically beneficial. The primary goal of this pilot study was to test the feasibility of using a novel robotic device –the RiceWrist Exoskeleton- for rehabilitation of the upper limbs (UL) of two tetraplegic persons with incomplete SCI. Two pilot experiments were conducted. Experiment 1was the first novel attempt to administer treatment with the RiceWrist. The left UL of a tetraplegic subject was treated during seven therapy sessions. The subject’s feedback and the investigator’s observations were used to enhance the robotic device and the corresponding graphical-interface. In Experiment 2, a second tetraplegic subject underwent 10 three-hour training sessions administered by a physical therapist. Smoothness factor (FS) –a new measure developed in Experiment 1- was used as the primary outcome to test the subject’s performance before and after the training. The RiceWrist was modified according to the feedback obtained in Experiment 1. Thereafter, the device was successfully administered for upper limb training of the tetraplegic individual. Noticeable improvements in FS were observed for the stronger arm of the subject who completed 10 sessions of training. Improvements were also observed in the subject’s hand according to the Jebsen-Taylor Hand Function Test. Results from this study suggest a potential application of the RiceWrist for rehabilitation of SCI individuals and offer valuable information regarding development of UL robotic devices for this population.
Keywords: Robotic Devices, Rice Wrist, Upper Extremity, Spinal Cord Injury
Article Outline
1. Introduction
- The annual incidence of spinal cord injury (SCI), not including those who die at the scene of injury, is approximately 12000 new cases each year[1]. The most frequent neurologic category at discharge of persons reported to the SCI Model Systems database has been incomplete tetraplegia (30.1%), followed by complete paraplegia (25.6%), complete tetraplegia (20.4%), and incomplete paraplegia (18.5%)[1]. Neurologically induced impairment of upper limbs (UL) is the rule following tetraplegia and results from paralysis or paresis of muscles[2]. According to a recent survey, more than 70% of tetraplegic individuals with SCIregarded UL function as an important or very important factor in their quality of life, exceeding concerns for sexual dysfunction (<50%), pain (<50%), and standing abilities (<45%)[3]. Only bowl and bladder problems were rated as equally or more important than regaining UL function[3]. These findings align with previous surveys that report UL function as the most important contributor to the quality of life of individuals with cervical SCI and therefore tetraplegia[4]. There is evidence that repeated and intensive practice can induce practice-dependent brain and spinal plasticity[5,6] and can result in significant UL improvement in persons with incomplete SCI[7]. Despite such promising findings, there are no established methods for delivering repeated practice to persons with SCI[8]. Robotic devices can help therapists deliver repeated practice and could potentially automate labour-intensive therapy procedures and lower therapy costs. Additional potential advantages of robotics include bringing therapy to new venues including the home, new sensing capabilities for monitoring progress, and increased therapy efficiency with the possibility of group therapy. Thus far, the dominant research effort for UL rehabilitation robotics has been the design of novel therapeutic robots or devices for stroke rehabilitation[9-11]. Despite growing literature on robotic UL training in stroke rehabilitation[10-12] only one pilot study has implemented shoulder and elbow robotic training (MIT-MANUS) for nine individuals with incomplete SCI[13]. The reported data from this pilot study were limited to improvements in Fugl-Meyer scores from two participants with no details on the modes of training or the subjects’ level of disability. The present study introduces the RiceWrsit robotic device as a new approach for delivering UL repeated practice to tetraplegic persons with SCI. This pilot study was conducted in the form of two experiments. Experiment 1 was the first novel attempt to administer RiceWrist robotic device for UL movements for a tetraplegic person with incomplete SCI. The primary goals of Experiment 1 were to: (1) test the feasibility of using the RiceWrist for an SCI individual while making necessary adjustments based on her feedback, and (2) quantify the subject’s performance with a newly developed robotic measure of smoothness (i.e. smoothness factor). Clinical application of the RiceWrist was tested in Experiment 2 as a physical therapist administered 10 sessions of UL robot-assisted training to another tetrapleic person with incomplete SCI. The smoothness factor developed in Experiment 1, and the Jebsen-Taylor Hand Function Test were used to detect robotic and functional changes in motor performance after the training.
2. Experiment 1
2.1. Participant
- A 27-year-old female with incomplete cervical SCI at the level of C2, with American Spinal Injury Association (ASIA) impairment scale C -according to ASIA Impairment Scale- 2 years post-injury, was recruited from The Institute for Rehabilitation and Research (TIRR) Memorial Hermann Hospital of Houston, Texas. Robot assisted movements were carried out for her left limb which exhibited a moderate level of weakness (ASIA score 18). She participated in 7 testing sessions with the RiceWrist robotic device after signing consent forms approved by the Institutional Review Boards of all involved institutions.
2.2. Apparatus
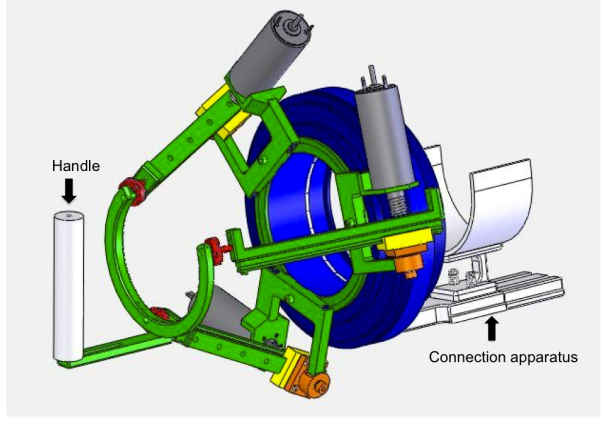
- The RiceWrist robotic device is an electrically actuated forearm and wrist exoskeleton designed and manufactured for rehabilitation purposes at Rice University. The mechanical design builds upon its predecessor, the MAHI Exoskeleton[14]. Joint-space as well as task-space position controllers and an impedance-based force controller for the device have been previously developed[15]. The exoskeleton is comprised of a revolute joint for forearm rotation and a 3-Revolute Prismatic Spherical (RPS) serial-in parallel wrist (Fig. 1).
2.3. Procedure
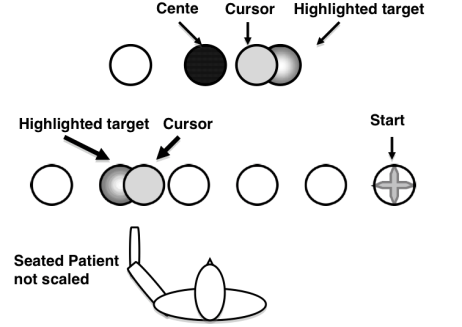
- During each session, the subject was seated behind a low table, centred in front of a computer monitor, with the left hand inside the robotic device holding the cylindrical handle of the device. The subject was seated comfortably in an upright position with the knees flexed at about 90°, trunk maintained against the back of the wheelchair, shoulder slightly abducted and elbow slightly flexed and forearm at the neutral position (midway between supination and pronation). An elastic bandage was used to wrap the subject’s hand due to her inability to maintain her grasp throughout the movement (Fig. 2).
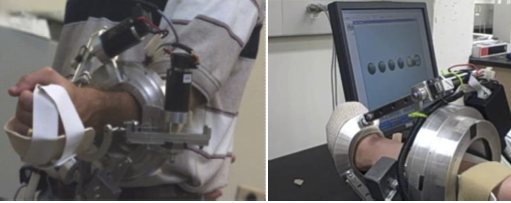 | Figure 2. RiceWrist worn by a healthy individual (left), The left hand of the SCI subject wrapped around the handle of the RiceWrist (Right) |
2.4. Primary Measures of Interest
- Smoothness of movement (SM) - The SM measure is a correlation coefficient that indicates the relationship between the patient’s velocity profile and a velocity profile based on the minimum jerk principle (an optimally smooth velocity profile). During discrete movements, the velocity profile of healthy persons’ movements can be represented by a profile that minimizes the squared jerk (the rate of change of acceleration). Optimally smooth velocity profiles can accurately represent discrete movements of the wrist[17,18], forearms[19] and arm[20]. The formulation developed by[21] that was also used by[17,22] was adopted for movement smoothness calculations. The velocity profile of the subject was derived from the angular velocity of the subject’s movements. The minimum jerk speed profile on a straight line for each target hit movement was calculated by equation (1),
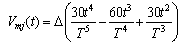 | (1) |
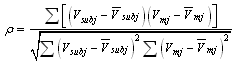 | (2) |
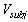 is the movement speed of the subject,
is the movement speed of the subject, 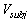 is the mean movement speed of the subject,
is the mean movement speed of the subject, 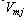 is the minimum jerk speed profile, and
is the minimum jerk speed profile, and 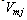 is the mean minimum jerk speed, following the formulation given in[17]. A correlation value of 1 indicates a perfect relationship to the minimum jerk profile. During data processing, negative correlation values occasionally calculated for individual movements, which implied negative correlation, were set to zero.Smoothness Factor (FS) - The smoothness factor is the product of ρ calculated from equation (2) and the coefficient of determination
is the mean minimum jerk speed, following the formulation given in[17]. A correlation value of 1 indicates a perfect relationship to the minimum jerk profile. During data processing, negative correlation values occasionally calculated for individual movements, which implied negative correlation, were set to zero.Smoothness Factor (FS) - The smoothness factor is the product of ρ calculated from equation (2) and the coefficient of determination 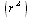 between the participant’s velocity profile and a fourth-order best-fit curve. This measure was developed due to limitations that were observed during calculation of SM, to be further explained in the following sections. Similar to SM, FS values of 1 indicate a perfectly smooth movement and occasional negative correlations were set to zero. Simulation- To clarify differences of SM and FS in representing the subject’s movement smoothness, two trajectories with different levels of oscillation, representing different velocity profiles, were generated as sine waves with corresponding minimum jerk profiles
between the participant’s velocity profile and a fourth-order best-fit curve. This measure was developed due to limitations that were observed during calculation of SM, to be further explained in the following sections. Similar to SM, FS values of 1 indicate a perfectly smooth movement and occasional negative correlations were set to zero. Simulation- To clarify differences of SM and FS in representing the subject’s movement smoothness, two trajectories with different levels of oscillation, representing different velocity profiles, were generated as sine waves with corresponding minimum jerk profiles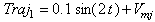 and
and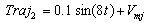 Where t is time and equals
Where t is time and equals  seconds and
seconds and  is the corresponding minimum jerk profile. Best-fit polynomials were fit to the simulated data and plotted along with the minimum jerk profile. SM and Fs values were calculated for the trajectories based on the formulations stated above.
is the corresponding minimum jerk profile. Best-fit polynomials were fit to the simulated data and plotted along with the minimum jerk profile. SM and Fs values were calculated for the trajectories based on the formulations stated above. 2.5. Results
- The subject completed 7 sessions of testing with the RiceWrist robotic device. All modifications that were made in response to the subject’s feedback and the investigators’ observations were completed in the first 5 sessions and are as follows:Customized splint: Immediately after Session 1, the subject expressed discomfort in her forearm due to its direct exposure with the RiceWrist forearm ring. As a result, a customized forearm splint made of thermoplastic material was designed and attached inside the forearm ring. The subject reported no discomfort or pain throughout the remaining sessions. The splint did not interfere with any of the robot-assisted movements.Graphical interface: All wrist and forearm movements were performed from a neutral forearm position. Therefore, wrist flexion/extension and radial/ulnar deviation occurred in horizontal and sagittal planes respectively. The original presentation of all targets in the horizontal plane complicated translation of wrist radial/ulnar deviation to a horizontal alignment. As a result, target display was modified to a vertical plane for radial/ulnar deviation for both target hitting and distortion games. The subject expressed satisfaction with this form of target alignment, and this configuration was used for all remaining sessions. Range of motion (ROM) calculation: The subject’s ROM for each movement direction was initially calculated before the start of each computer game by means of the target hitting interface in the active-constraint mode with zero constraint. Originally, the subject was asked to move to targets at each end where ROM was registered as one value for every plane of movement from one maximum point to another (e.g. one ROM value for flexion/extension in the horizontal plane calculated from maximum flexion to maximum extension). This approach did not provide distinct ROM values for opposite movement directions within each plane of movement (e.g. flexion vs. extension). To overcome this limitation, ROM calculation was modified and each movement direction was registered separately from the neutral position. Therefore, the distance of each target from the centre represented corresponding ROM values for each movement direction.Counterweight selection: The wrist component of the RiceWrist employs a 3-RPS parallel mechanism where the links of the mechanism are actuated by three electrical motors fixed on the base plate (see Fig. 1). Because of the asymmetrical configuration of the motors, an appropriate counterweight is required to maintain the moment balance for the forearm motions that would allow the forearm movement to occur as in free space. Throughout initial sessions it was observed that another important factor to consider for choosing a suitable counterweight was the configuration of the power and encoder cables of the motors. Consequently the counterweight was increased after accounting for all contributing factors allowing for the forearm movement to occur as in free space.
|
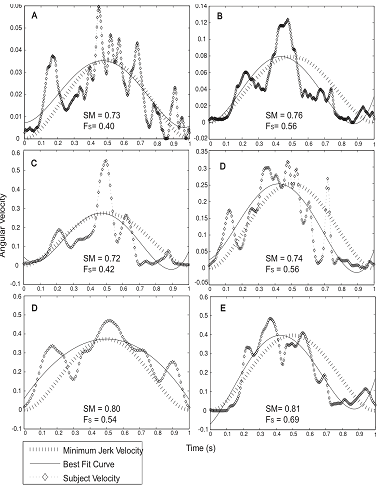 | Figure 4. Angular velocity profiles of (A) forearm supination, (B) wrist radial deviation and (C) wrist extension with corresponding movement smoothness (SM) and smoothness factor (Fs) values calculated for performances during visible condition of distortion game |
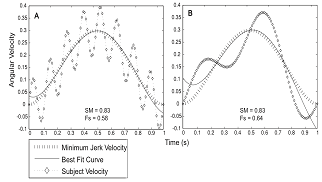 | Figure 5. Simulated trajectories are depicted with different levels of oscillation and corresponding movement smoothness (SM) and smoothness factor (Fs) values. Both trajectories are over one second, and the targets are one unit apart. Thus, the minimum jerk profile is the same for the two plots |
2.6. Discussion
- The present study was the first attempt to successfully administer and customize an exoskeleton robotic device, the RiceWrist, for delivering distal UL movements to a person with incomplete SCI. Use of an exoskeleton was considered because of the many advantages it holds over end-effector based robots. End-effector based robots such as MIT-MANUS[24], a planar manipulator with a workspace in the horizontal plane, and the MIME, based on an industrial robot[11], provide training capabilities encapsulating a large portion of the functional workspace. However, end-effector robots do not possess the ability to control specific joints. Exoskeletons such as RiceWrist, Rupert[25], ARMin[26] and CADEN-7[27] are designed to resemble human anatomy and their structure enables individual actuation of joints. RiceWrist and other exosketons offer the advantage of precisely recording and monitoring isolated joint movements as depicted by recorded measures of smoothness collected for wrist extension, radial deviation, and forearm supination in the current study (Figures 4 and 5). Furthermore, given that muscles of the affected limbs and therefore movement capabilities at each joint often demonstrate different levels of weakness after incomplete SCI[28], exoskeletons are better suited than end-effector based designs for rehabilitation of persons with SCI. For our subject, the preferred choice of the active-constraint mode at zero constraint was because of her inability to perform singular wrist and forearm movements free of unwanted compensatory activities that occurred when movements were attempted without assistance. Compensatory movements are secondary strategies that normally occur as a result of weakness[18,29] and, in the case of our subject, included radial deviation and forearm supination when attempting wrist extension, and trunk lateral flexion when attempting forearm supination. With the RiceWrist, the forearm joint was constrained during wrist movements. Furthermore, only movements in the direction of interest could move the cursor on the screen, and with the hand strapped in the RiceWrist, any attempted trunk compensations did not trigger wrist or forearm movements. Movement smoothness was the primary robotic measure calculated for recorded wrist and forearm activities of the SCI subject who participated in this study. Movement smoothness has been used to determine motor performance of healthy individuals[30] and persons who have suffered stroke[17,21,22,31]. Smoothness measures are often based on minimum jerk (third time derivative of position) or snap (fourth time derivative of position) as introduced by[32,33] respectively. However, third or fourth time derivatives of position introduce excessive noise and eliminate useful content. Therefore, calculation of movement smoothness has been based on the formulation in[21] as the correlation between the subject’s velocity profile and the optimally smooth speed profile (SM) (similar to the techniques of[17-22]). Our approach in calculating FS revealed more sensitivity to oscillations in the subject’s movements as represented by distinct levels of smoothness that were undetectable by SM (Fig. 4). Simulated trajectories further confirmed these findings, where despite evidently different oscillations of the two trajectories around the minimum jerk profile, they could be considered equally “smooth” when compared according to SM (p=0.083). FS accurately reflected the distinction of simulated trajectories. Other well-known measures of smoothness have been presented by[34], including speed-metric, mean arrest period ratio and peak metric. While these measures appear to successfully detect movement smoothness in persons with stroke, they are specific to episodic movements reflected by several stops or near stops during a performance (i.e. submovements). Observation of individual profiles in our study revealed that complete stops were not always present during wrist and forearm movements, despite high levels of movement oscillations (Fig 4D-E). The observed velocity profiles are similar to a motion-capture study where shoulder extension velocity profiles of a tetraplegic SCI patient were highly oscillatory during actively performed hand to neck movement with no evident stops throughout the movement[35]. Hence, given that the majority of existing robotic measures have been developed for persons with stroke, these measures should be carefully examined before they are used for persons with SCI.
3. Experiment 2
- With successful application of the RiceWrist in the above case, Experiment 2 was designed to demonstrate clinical application of the RiceWrist for robot-assisted UL training of right and left arms of a tetraplegic person with incomplete SCI. The robotic measure FS and the clinical measure Jebsen-Taylor Hand Function Test (JTHFT) were used to compare motor performance before and after training.
3.1. Participant
- A 24-year-old male with incomplete SCI at the C4 level, ASIA impairment scale D, 6.5 months post-injury was recruited from TIRR of Houston. He participated in 10 sessions of robotic training over 2 weeks. Minimum voluntary movements were preserved on the right upper extremity-weaker limb (ASIA score 8), whereas on the left side he had moderate level of voluntary movement-stronger limb (ASIA score 23). He signed a consent form approved by the Institutional Review Boards of all involved institutions.
3.2. Procedure
- Robotic training was provided for the right and left limbs with the RiceWrist for three hours per day on 10 consecutive weekdays. The experimental set-up including the subject’s position arrangements, and the robotic device settings were comparable to Experiment 1 (see Fig 1 and 2). Evaluation trials were completed for the left hand (stronger hand) followed by the opposite hand in Sessions 1 and 10 for pre- and post- comparisons. The evaluation trial involved administration of the clinical JTHFT test by a physical therapist, and robotic movement smoothness assessment using the RiceWrist. A series of target hitting tasks, conducted via a computer-based graphical display as shown in Fig. 2, were carried out by flexion/extension, radial/ulnar deviation or forearm supination/pronation, enabling robotic evaluation. The distance of the two targets from the centre was based on the subject’s maximum range of motion that was captured with the RiceWrist as described earlier. The subject performed 20 target hits for each plane of movement in the active-constraint mode with zero constraint during evaluations. Training followed evaluation and involved target hitting and distortion tasks, each tailored individually based on the subject’s movement capabilities. The target-hitting task was the same as evaluation with the exception that all three operating modes (passive, active-constraint and triggered) were administered. The number of repetitions and speed of movement were provided to the subject as visual feedback throughout his performance for motivational purposes. Task difficulty was increased by gradually adding to the number of repetitions. The amount of applied resistance and threshold level during active-constraint and triggered modes were gradually increased to add to the difficulty of the task. All training and evaluations were administered by a physical therapist.
3.3. Primary Measures of Interest
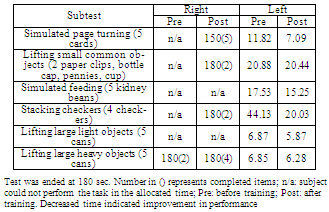
- Robotic measure - Fs was calculated from angular position data collected at 100 Hz for all evaluation trials. This measure was calculated as described for Experiment 1.Clinical Measure – Jebsen-Taylor Hand Function Test (JTHFT)[36], is a measure of function rather than movement and was selected as the clinical measure of interest. This test has been used extensively and successfully in the spinal cord injury populations[7] and includes various functional tasks such as turning cards, feeding using a teaspoon, lifting small, large and heavy objects and stacking cards. These tasks are designed to mimic functions used during activities of daily living. The time to complete each task is recorded and compared. A physical therapist administered JT before and after the training to assess functional improvements in upper limbs.
3.4. Results
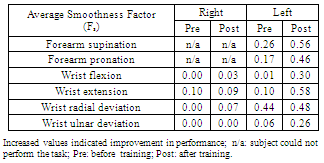
- The subject was able to successfully complete 10 sessions of robot assisted training. While evaluation trials were completed for all movements with the left upper limb, this was not the case for the right upper limb. The participating individual was unable to voluntarily perform forearm supination and pronation with the right limb due to severe weakness. Hence, no evaluation trails were completed for these movement directions, and training was only operated in the assistive mode. For the same reason, the subject was unable to perform several tasks of the JTHFT with the right upper limb during initial assessments that took place before training. In order to compare movement smoothness before and after training, evaluation data from Sessions 2 and 10 were used for comparison. Data collected in the first training session were discarded due to the subject’s unfamiliarity with the task and his inability to adhere to the provided instructions during this session. As presented in Table 3, comparison of average FS values for the left upper limb before and after training indicated a considerable increase for all movements. The smallest improvement in FS was observed for the wrist radial deviation.
|
|
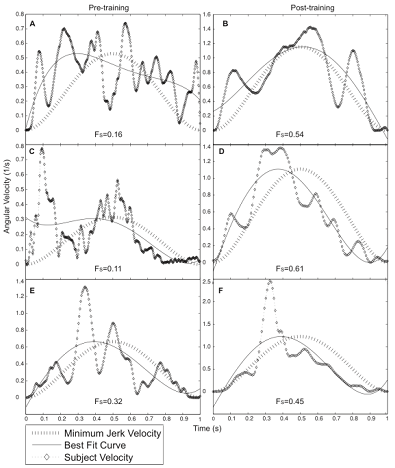 | Figure 6. Angular velocity profiles of a single target hit for forearm pronation (A,B), wrist extension (C,D) and wrist radial deviation (E,F) before (left panel-A,C) and after (right panel-B,D) robotic training for the left upper limb. Corresponding smoothness factor values (Fs), minimum jerk velocity profiles and the best fit curves are also presented. Pre: before training; post: after training; Fs: smoothness factor |
3.5. Discussion
- In the present study, the clinical application of the RiceWrist for a tetraplegic person with incomplete SCI was successfully completed in the course of 10 sessions of UL training for moderate (left hand) and severe (right hand) levels of impairment. Three-hour training sessions were delivered on consecutive weekdays over two weeks, and involved repeated practice of singular wrist and forearm movements.The intensive training schedule was based on the training principle of “overload” suggested for maximizing training effects[37]. The repeated practice addressed the subject’s impairment in performing discrete movements according to the “specificity of practice” suggesting for practice to be the same as the targeted skill[38]. Findings indicated considerable motor progress for the left UL evident by the gains in movement smoothness for wrist flexion/extension and forearm supination/pronation (Table 3). No substantial changes were observed in the smoothness measures of the left wrist radial deviation, and the right wrist and forearm movements (Table 3). What has been reported to date for tetraplegic persons with SCI is the ttransformation of an oscillatory velocity to a single peaked smooth profile after surgical interventions[35], but no direct measures of smoothness were calculated. To our knowledge, no other studies have looked at movement smoothness throughout therapy for the tetraplegic individuals who have suffered incomplete SCI. Hence, our preliminary results for SCI robotic rehabilitation are novel in contrast to the extensive reports of increased UL movement smoothness for persons with stroke after robot-assisted[12,17,39], and traditional treatments[40,41]. Unlike stroke, it is unclear what mechanisms deteriorate movement smoothness in persons with SCI[31]. Normal agonist, antagonist muscle activation[42] and intact cortical planning[31] are suggested to be important for generating ideally smooth movements. While abnormal neural coordination (spasticity)[43,44] and secondary cortical degenerations[45] have been observed in persons with cervical SCI, it is not clear whether these mechanisms were involved in the movement smoothness values observed before and after the current training.Improvements in JTHFT were evident for the right and left limbs but, they were not consistent across all subtests and were greatest for simulated page turning and stacking checkers (see Table 4). JTHFT is a time based test and does not capture changes in adopted motor strategies. However, given that compensatory movements that commonly occur as a result of UL weakness[18,29] were not possible during robot-assisted training, improvement in JT might have been a reflection of better control strategies as a result of training. Further studies are required to confirm such an assumption. Together, FS and JT observations suggest a less prominent improvement for the right UL in the kinematic and functional contexts. The initial capabilities of denervated muscles highly influence the speed and magnitude of their sensory-motor recovery with faster recovery for higher functioning muscles[46]. The observation that the subject’s right upper limb was more severely affected as indicated by the lower ASIA score and the subject’s inability to actively perform supination and pronation could explain the limited capabilities of the right UL observed after training. This lack of improvement may be suggestive of the need for alternative or longer forms of therapy. Several studies have indicated that for tetraplegic persons with incomplete SCI, massed practice is more effective when combined with sensory stimulations (e.g. functional electrical stimulation) than when delivered alone[7,47]. These studies also suggest a minimum training dose of 15 sessions for effective training results. Therefore, it is possible that longer or combined forms of therapy may have induced greater effects for cases with limited improvements. It should be noted that the effects of spontaneous recovery could not be ruled out for the clinical and robotic improvements of our subject who was only 6.5 months post injury. The majority of spontaneous recoveries occur in the first three months after the injury with smaller and slower improvements up to 18 months post injury[46,48]. That said, and given evidence of enhancements in neural plasticity with massed practice[5], we cannot disregard the positive effect of the administered robot-assisted training. Present findings confirm the great potentials of rehabilitation robots in delivering therapy to persons with SCI and other disabilities that may benefit from repeated practice.
4. Conclusions
- Experiments 1 and 2 suggest that robotic devices can potentially play a critical role in the rehabilitation of persons with SCI. Robotic measures collected from conducted experiments further imply a potential use of the RiceWrist for motor assessment of persons with SCI. Many methods of motor evaluation currently used by clinicians are based on numerical scoring systems (e.g. Capabilities of Upper Extremities[49] and Tetraplegic Hand Questionnaire[50], or timing tests (Jebsen-Taylor Hand Function[36]). These measures lack direct physical meaning and this critical limitation influences accurate characterization of existing impairments or sensori-motor changes that occur as a result of therapy[8,51]. Robotic-devices such as the RiceWrist, and robotic measures such as FS, can provide and serve as outcome measures not derivable from common forms of assessment. The current study was pilot work with a limited number of subjects. We acknowledge this limitation and our primary use of active-constraint mode for the reported robotic outcome. Further investigations are underway to not only use outcome measures collected from other operating modes, but to also include larger SCI populations with different levels of disability.
ACKNOWLEDGEMENTS
- The Authors acknowledge Mission Connect, a project of TIRR Foundation, and H133P0800007-NIDRR-ARRT. We also thank our subjects for their valuable participation.
References
| [1] | Spinal Cord Injury association, A.S.I., Reference Manual of the International Standards for Neurological Classification of Spinal Cord Injury, in Chicago, IL. 2003, American Spinal Injury Association. |
| [2] | Kirshblum, S.C. and K.C. O'Connor, Predicting neurologic recovery in traumatic cervical spinal cord injury. Saunders publishing, Arch Phys Med Rehabil, vol.79, no.11, pp.1456-66, 1998. |
| [3] | Snoek, G.J., IJzerman, M.J., Hermens, H.J., Maxwell, D, Biering-Sorensen, F., Survey of the needs of patient.s with spinal cord injury: impact and priority for improvement in hand function in tetraplegics. Mcmillan publishing, Spinal Cord, vol.42, no.9, pp.526-32, 2004. |
| [4] | Hanson, R.W. and M.R. Franklin, Sexual loss in relation to other functional losses for spinal cord injured males. Saunders publishing, Arch Phys Med Rehabil, vol.57, no.6, pp.291-3, 1976. |
| [5] | Hoffman, L.R. and E.C. Field-Fote, Functional and corticomotor changes in individuals with tetraplegia following unimanual or bimanual massed practice training with somatosensory stimulation: a pilot study. APTA publishing, J Neurol Phys Ther, vol.34, no.4, pp.193-201, 2010. |
| [6] | Lynskey, J.V., A. Belanger, and R. Jung, Activity-dependent plasticity in spinal cord injury. RRDS publishing, J Rehabil Res Dev, vol.45, no.2, pp.229-40, 2008. |
| [7] | Beekhuizen, K.S. and E.C. Field-Fote, Massed practice versus massed practice with stimulation: effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Demos Medical Pub publishing, Neurorehabil Neural Repair, vol.19, no.1, pp.33-45, 2005. |
| [8] | Mulcahey, M.J., D. Hutchinson, and S. Kozin, Assessment of upper limb in tetraplegia: considerations in evaluation and outcomes research. publishing, RRDS publishing, J Rehabil Res Dev, vol.44, no.1, pp. 91-102, 2007. |
| [9] | Hogan, N., Impedance control: an approach to manipulation: Part Itheory, Part II-implementation, Part III- applications. IEEE publishing, Journal of Dynamic System Measurement and Control. vol.107, pp. 1024, 1985. |
| [10] | Krebs, H.I., Volpe B.T., Williams, D., Celestino, J., Charles, S.K., Lynch, D. and Hogan, N., Robot-aided neurorehabilitation: a robot for wrist rehabilitation. IEEE Piscataway publishing, Trans Neural Syst Rehabil Eng, vol.15, no.3, p. 327-35, 2007. |
| [11] | Lum, P.S., Burgar, C.G., Shor, P.C., Majmundar, M., and Van der Loos, M., Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Saunders publishing, Arch Phys Med Rehabil, vol.83, no.7, pp. 952-9, 2002. |
| [12] | Colombo, R., Pisano, F., Micera, S., Mazzone, A., Delconte, C., Carrozza, M.C., Dario, P., Minuco, G., Assessing mechanisms of recovery during robot-aided neurorehabilitation of the upper limb. Thousand Oak publishing, Neurorehabilitation and Neural Repair, 2008. 22(1): p. 50-63. |
| [13] | Krebs, H.I., Dipietro L., Levy-Tzedek, S., Fasoli, S.E., Rykman-Berland, A., Zipse, J., Fawcett, J.A., Stein, J., Poizner, H., Lo, A.C., Volpe, B.T., and Hoganet, N., A paradigm shift for rehabilitation robotics: therapeutic robots enhance clinician productivity in facilitating patient recovery. EMB publishing, IEEE Eng Med Biol Mag. vol.27, no. 4, pp.61-70, 2008. |
| [14] | Gupta, A. and M.K. O'malley, Design of a haptic arm exoskeleton for training and rehabilitation. IEEE publishing, IEEE-ASME Transactions on Mechatronics, vol. 11, no.3, pp. 280-289, 2006. |
| [15] | Gupta, A., O'Malley, M.K., Patoglu, V., Burgar, C., Design, Control and Performance of RiceWrist: A Force Feedback Wrist Exoskeleton for Rehabilitation and Training. SAGE publishing, The International Journal of Robotics Research vol.27, no.2, pp. 233-251, 2008. |
| [16] | Brewer, B.R., R. Klatzky, and Y. Matsuoka, Visual feedback distortion in a robotic environment for hand rehabilitation, Elsevier Science publishing, Brain Res Bull, vol.75, no.6, pp. 804-13, 2008. |
| [17] | Celik, O., O'Malley, M.K., Boake, C., Levin, H.S., Yozbatiran, N., Reistetter, T.A., Normalized Movement Quality Measures for Therapeutic Robots Strongly Correlate With Clinical Motor Impairment Measures. EMB publishing, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol.18, no.4, pp. 433-444, 2010. |
| [18] | Hoffmann, G., Laffont, I., Hanneton, S., Roby-Brami, A., How to extend the elbow with a weak or paralyzed triceps: control of arm kinematics for aiming in C6-C7 quadriplegic patients. Elsevier Science publishing, Neuroscience, vol.139, no.2, pp. 749-65, 2006. |
| [19] | Huegel, J.C., A. Lynch, and M.K. O’Malley, Validation of a smooth movement model for a human reaching task. In 2009, Proceedings of IEEE International Conference on Rehabilitation Robotics, pp. 799-804, 2009. |
| [20] | Liebermann, D.G., et al., Arm path fragmentation and spatiotemporal features of hand reaching in healthy subjects and stroke patients. In 2010, Conf Proc IEEE Eng Med Biol Soc. pp. 5242-5, 2010. |
| [21] | Krebs, H.I., Volpe, B.T., Palazzolo, J., Rohrer, B., Ferraro, M., Fasoli, S., Edelstein, L., Hogan, N. Robot-Aided Neuro-Rehabilitation in Stroke: Interim Results on the Follow-up of 76 Patients and on Movement Performance Indices. in 2001, proceedings of IEEE International Conference on Rehabilitation Robotics, 2001. |
| [22] | Daly, J.J., Hogan, N., Perepezko, E.M., Krebs, H.I., Rogers, J.M., Goyal, K.S., Dohring, M.E., Fredrickson, E., Nethery, J., and Ruff, R.L. Response to upper-limb robotics and functional neuromuscular stimulation following stroke, RRDS publishing, J Rehabil Res Dev, vol.42, no.6, pp. 723-36, 2005. |
| [23] | Perry, J.C., Rosen, J., and Burns, S., Upper-limb powered exoskeleton design, IEEE publishing, IEEE/ASME Transactions on Mechatronics. vol.12, no.4, pp. 408-417, 2007. |
| [24] | Krebs, H.I., Hogan, N., Aisen, M.L., Volpe, B.T., Robot-aided neurorehabilitation, IEEE EMB publishing, IEEE Transactions on Rehabilitation Engineering, vol.6, no.1, pp. 75-87, 1998. |
| [25] | Sugar, T.G., He, J., Koeneman, E.J., Koeneman, J.B., Herman, R., Huang, H., Schultz, R.S., Herring, D.E., Wanberg, J., Balasubramanian, S., Swenson, P., Ward, J.A., Design and control of RUPERT: a device for robotic upper extremity repetitive therapy. IEEE publishing, IEEE Trans Neural Syst Rehabil Eng, vol.15, no.3, pp. 336-46, 2007. |
| [26] | Nef, T., Mihelj, M., Kiefer, G., Perndl, C., Muller, R., Riener, R., ARMin - Exoskeleton for Arm Therapy in Stroke Patients. IEEE Proceedings of 10th Internatinal Confernce on Rehabilitation Robotics, pp. 68-74, 2007. |
| [27] | Rosen, J., and J.C. Perry, Upper limb powered exoskeleton. International Journal of Humanoid Robotics, vol.4, no.3, pp. 529-548, 2007. |
| [28] | Thomas, C.K., Zaidner, E.Y., Calancie, B., Broton, J.G., and Bigland-Ritchie, B.R., Muscle weakness, paralysis, and atrophy after human cervical spinal cord injury. Academic Press publishing, Exp Neurol, vol.148, no.2, pp. 414-23, 1997. |
| [29] | Baba, M., M. Matsunaga, and I. Ozaki, Posterior interosseous syndrome in acute Guillain-Barre syndrome, Nauwelaerts Publishing, Electromyogr Clin Neurophysiol, vol.34, no.6, pp. 367-71, 1994. |
| [30] | Platz, T., Denzler, P., Kaden, B., Mauritz, K.H., Motor learning after recovery from hemiparesis. ELSEVIER Science publishing, Neuropsychologia, vol.32, no.10, pp. 1209-23, 1994. |
| [31] | Tsao, C.C. and M.M. Mirbagheri, Upper limb impairments associated with spasticity in neurological disorders. Biomed Central publishing, J Neuroeng Rehabil, vol.29, no.4, pp. 45, 2007. |
| [32] | Flash, T. and N. Hogan, The coordination of arm movements: an experimentally confirmed mathematical model, SFN publishing, J Neurosci, vol.5, no.7, pp. 1688-703, 1985. |
| [33] | Edelman, S. and T. Flash, A model of handwriting, Springer Verlag publishing, Biol Cybern, vol.57, no.1-2, pp. 25-36, 1987. |
| [34] | Rohrer, B., Fasoli, S., Krebs, H.I., Hughes, R., Volpe, B., Frontera, W.R., Stein, J., Hogan, N., Movement smoothness changes during stroke recovery. SFN publishing, J Neurosci, vol.22, no.18, pp. 8297-304, 2002. |
| [35] | Remy-Neris, O., Milcamps, J., Chikhi-Keromest, R., Thevenon, A., Bouttens, D., Bouilland, S., Improved kinematics of unrestrained arm raising in C5-C6 tetraplegic subjects after deltoid-to-triceps transfer, tockton Press publishing, Spinal Cord, vol.41, no.8, pp. 435-45, 2003. |
| [36] | Jebsen, R.H., Taylor, N., Trieschmann, R.B., Trotter, M.J., Howard, L.A., An objective and standardized test of hand function. Saunders publishing, Arch Phys Med Rehabil, vol.50, no.6, pp. 311-9, 1969. |
| [37] | Kisner, C., Therapeutic Exercise: Foundations and Techniques. 2 ed. Philadelphia: Pa: FA Davis Co, 1990. |
| [38] | Magill, R.A., Introduction to Motor Skill Learning, in Motor Learning and Control, E. Barrosse, Editor. McGraw-Hill: New York. pp. 265-281, 2007, |
| [39] | Colombo, R., Sterpi, I., Mazzone, A., Delconte, C., Minuco, G., Pisano, F., Measuring Changes of Movement Dynamics During Robot-Aided Neurorehabilitation of Stroke Patients. IEEE publishing, IEEE Transactions on Neural Systems and Rehabilitation Engineering, vol.18, no.1, pp. 75-85, 2010. |
| [40] | Lin, K.C., Wu, C.Y., Wei, T.H., Lee, C.Y., Liu, J.S., Effects of modified constraint-induced movement therapy on reach-to-grasp movements and functional performance after chronic stroke: a randomized controlled study, SAGE publishing, Clin Rehabil, vol.21, no.12, pp. 1075-86, 2007. |
| [41] | Caimmi, M., Carda, S., Giovanzana, C., Maini, E.S., Sabatini, A.M., Smania, N., Molteni, F., Using kinematic analysis to evaluate constraint-induced movement therapy in chronic stroke patients, Demos Medical Publishing, Neurorehabil Neural Repair, vol.22, no.1, pp. 31-9, 2008. |
| [42] | Krylow, A.M. and W.Z. Rymer, Role of intrinsic muscle properties in producing smooth movements. IEEE publishing, IEEE Trans Biomed Eng, vol.44, no.2, pp. 165-76, 1997. |
| [43] | Haisma, J.A., van der Woude, L.H., Stam, H.J., Bergen, M.P., Sluis, T.A., Post, M.W., Bussmann, J.B., Complications following spinal cord injury: occurrence and risk factors in a longitudinal study during and after inpatient rehabilitation. Taylor & Francis publishing, J Rehabil Med, vol.39, no.5, pp. 393-8, 2007. |
| [44] | McDonald, J.W., Spinal-cord injury, Lancet publishing, The Lancet, vol.359, pp. 417-425, 2002. |
| [45] | Davey, N.J., Smith, H.C., Wells, E., Maskill, D.W., Savic, G., Ellaway, P.H., Frankel, H.L., Responses of thenar muscles to transcranial magnetic stimulation of the motor cortex in patients with incomplete spinal cord injury. J Neurol Neurosurg Psychiatry, BMJ Publishing, vol.65, no.1, pp. 80-7, 1998. |
| [46] | Fawcett, J.W., Curt, A., Steeves, J.D., Coleman, W.P., Tuszynski, M.H., Lammertse, D., Bartlett, P.F., Blight, A.R., Dietz, V., Ditunno, J., Dobkin, B.H., Havton, L.A., Ellaway, P.H., Fehlings, M.G., Privat, A., Grossman, R., Guest, J.D., Kleitman, N., Nakamura, M., Gaviria, M., Short, D., Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials, Stockton Press publishing, Spinal Cord, vol.45, no.3, pp. 190-205, 2007. |
| [47] | Needham-Shropshire, B.M., Broton, J.G., Cameron, T.L., Klose, K.J., Improved motor function in tetraplegics following neuromuscular stimulation-assisted arm ergometry, Maney publishing, J Spinal Cord Med, vol.20, no.1, pp. 49-55, 1997. |
| [48] | Ditunno, J.F., Jr., Stover, S.L., Freed, M.M., Ahn, J.H., Motor recovery of the upper extremities in traumatic quadriplegia: a multicenter study, Saunders publishing, Arch Phys Med Rehabil, vol.73, no.5, pp. 431-6, 1992. |
| [49] | Marino, R.J., J.A. Shea, and M.G. Stineman, The Capabilities of Upper Extremity instrument: reliability and validity of a measure of functional limitation in tetraplegia, Saunders publishing, Arch Phys Med Rehabil, 1998. vol.79, no.12, pp. 1512-21, 1998. |
| [50] | Land, N.E., Odding, E., Duivenvoorden, H.J, Bergen, M.P., Stam, H.J., Tetraplegia Hand Activity Questionnaire (THAQ): the development, assessment of arm-hand function-related activities in tetraplegic patients with a spinal cord injury, Stockton Press, Spinal Cord, 2004. vol.42, no.5, pp. 294-301. |
| [51] | Tennant, A., Measuring outcome, Oxford University Press publishing, Br Med Bull, vol.56, no.2, pp. 287-95, 2000. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML