-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biological Engineering
p-ISSN: 2163-1875 e-ISSN: 2163-1883
2012; 2(2): 1-8
doi: 10.5923/j.ijbe.20120202.01
Cardio Vascular Grafts: Existing Problems and Proposed Solutions
Kunal Singha 1, Mrinal Singha 2
1Department of Textile Technology, Panipat Institute of Engineering & Technology, Harayana, India
2Department of Pharmaceutical Chemistry, CU Shah College of Pharmacy & Research, Gujarat, India
Correspondence to: Kunal Singha , Department of Textile Technology, Panipat Institute of Engineering & Technology, Harayana, India.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The cardiovascular diseases are becoming a major fear in the present era all over the globe. The cardiovascular tissue engineering (TE) can become an ideal substitute to replace all of this cardiac problems like: cardiovascular grafting, valves, microvessel construction, aorta and vein formation by using of some regularly used textile fibers like: ePTFE, PGA, nylon, PET (Dacron), polygalactin etc. this artificial health devices are fully bio-compatible, bio-degradable within the human body and also FDA approved. In this current review paper the existing problems related to the different cardiovascular grafts, their flow mechanics inside the human body, microvessel building engineering and their ideal examples has been extensively discussed.
Keywords: cardiovascular grafting, valves, microvessel construction, aorta and vein formation; ePTFE, PGA, nylon, PET (Dacron), polygalactin; bio-compatible, bio-degradable; flow mechanics; microvessel building engineering
Article Outline
1. Introduction
- Galen, the second century Greek physician believed to be the father of Western experimental physiology, conducted anatomical research almost entirely on apes and pigs to solves different heart and blood problems of human body. He extrapolated these findings to humans, which unfortunately initiated many errors. This brings us to the term ‘animal model’ or in actuality the modelling of human tissue[1]. Diseases of the heart and blood vessels are the largest cause of mortality in the USA and Europe. Furthermore, these disease processes cause much suffering because of pain, disability and limb loss due to peripheral vascular disease (PVD). Therefore there is a massive need for bypass grafts in both the coronary artery and lower limb circulations. For the majority of interventions this involves harvesting of the long saphenous vein from the leg or even the cephalic and basilic veins from the arm of the patient. However, in about a third of patients this proves inadequate or unsuitable. Therefore surgeons have turned to prosthetic materials like polyethylene terephthalate (Dacron), polytetrafluoroethylene (PTFE), expanded PTFE (ePTFE) and polyurethane. However, these prosthetic materials prove to be inferior to autologous conduits, especially when the vessel diameter is less than 5mm[2,4]. The problems include increased risk of thrombosis and infection, limited durability, lack of compliance both of the graft and around the anastomosis, and failure due to restenosis, thus necessitating further interventions. Currently several groups are working towards the development of ‘living grafts’, seeded grafts and hybrid grafts.Every year diseases of the heart valves result in 60,000 patients in the USA and 6,000 patients in the UK undergoing valve replacement surgery. The current options include mechanical and bioprosthetic heart valves. However, both have their shortcomings. Mechanical valves have increased risks of infection and require lifelong anticoagulant therapy to prevent thromboembolic events, but then these patients also suffer from the risks of excess bleeding. Bioprosthetic grafts do not have the same thromboembolic risk, but are less durable-failing via a multifactorial aetiology: inflammation; inability to effectively grow and autorepair; metabolic processes leading to infections as well as a gradual release of glutaraldehyde. This complex series of reactions in bioprostheses leads to a stepwise calcification causing a loss in haemodynamic performance resulting in their failure after a myocardial infarction[3]. Currently the only options include treatment of risk factors and a plethora of drugs which can be replaced by the tissue engineering (TE) which applies the principles of biology and engineering to develop functional substitutes for damaged tissue, such as generating new cardiac tissue, blood vessels, cardiac tissue and heart valves—become commercially available with the proper FDA approval prior to a clinical application[5-6].The aim of this review is to assess the types and models of graft chosen for TE for vascular constructs. Furthermore this review describes the usage of tested constructs for effectiveness, functionality and biodegradability and preliminary usages of sources of scaffolds for TE.
1.1. Animals generally used for search method in cardiovascular
- Porcine, canine, lapine, murine, pig, dog, horse, goat, cow, mouse, rat, rabbit, hamster, guinea pig, cat, monkey, primate (baboons) are generally used for the experimental search method for cardiovascular therapy. Our target to find out what kind of polymer; TE; cellular engineering; graft, coronary; myocytes; vascular; dialysis; bypass; heart valve; heart; cardiac can be suitable used into the human body in–vivo without any side-effects or dangers 7-10].
1.2. Why we need vascular graft
- i Substituting damage blood vessel of the human body- due to artificial tissue damage like in burning- here we can use dermal substitute- Integra[11].ii To replace injured blood vessel, artery, vein etc[8,12].iii Substitute the stenosis or hardening of blood vessels, heart valves in the older patients.iv To surgery the autogenous, saphenous veins, standard coronary and infrapopliteal bypass to many patients do not have suitable vein[13-15].v Small-caliber vascular xenograft[16].
2. How we can do vascular graft: the ways to modelling the actual blood circulation inside the human body
- i In current practice, reconstructive surgery is essential in order to achieve wound closure following major cancer resections and trauma. Traditionally, reconstructive surgeons have employed the reconstructive ladder concept; a hierarchical system, by which the simplest option, usually skin grafting or primary closure, is chosen first. If this fails, then a slightly more complex procedure is selected[17]. With the increasing demand for quality wound closure, maximal aesthetics with minimum morbidity, the concept of the ‘reconstructive elevator’ is applicable. As opposed to the reconstructive ladder, the best option, which in most cases is a flap is chosen initially. A flap may be defined as a segment of tissue with an independent blood supply. These maybe classified into local, regional and free flaps. Free flaps are based on the concept of angiosome; an area of tissue with an inherent vascular network supplied by a single vascular pedicle[18]. ii Buncke and McLean performed the first microvascular tissue transplant in 1969. Since then, free flaps have revolutionised reconstructive surgery as they are more versatile and reliable than other flaps. Flaps are now being pre-fabricated prior to surgery to become more case-specific[19]. More recently, the introduction of perforator flaps[20] has improved the utility of flaps as a reconstructive option. Nevertheless, free tissue transfer confers morbidity to the patient as one function is sacrificed for another. Therefore, alternative sources of tissue for reconstruction are needed.
3. Microvessel developments
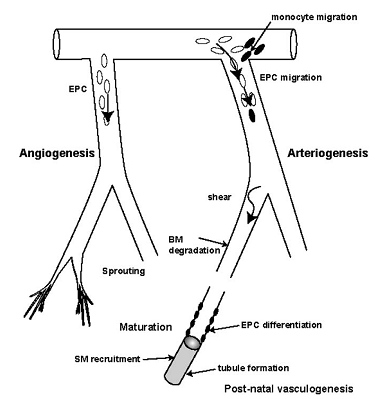
- Human micro vasculature begins with arteries dividing consecutively into smaller branches like meta-arterioles (80–100μm) until finally forming capillaries (10–15μm). These vessels serve to redistribute blood and its nutrients whilst lowering the pressure head[21]. This allows blood to perfuse the tissue, allowing more efficient exchange of metabolites.The vascular tree is formed during the early gestation. Angiogenic cells form clusters which coalesce to form solid tubes, which eventually canalise to form blood vessels. The outer ring consists of angioblasts which form the vessel walls. The subsequent differentiation of these precursor angioblasts into endothelial cells (EC) and the denovo formation of a vascular network are termed ‘vasculogenesis’[23-25]. These vessels are capillary-like to begin with and eventually differentiate into either arteries or veins. The creation of this framework depends on guidance molecules within the matrix such as ephrins at the arterio-venous interface and reversion-inducing cysteine-rich (RECK) protein for paracellular proteolysis[22].The adult vascular network remodels itself by arteriogenesis with the opening up and then the subsequent enlargement of existing collaterals (so-called collateral enlargement), as well as the formation of completely new vessels from the already existing vessels (so-called arterialisation). Micro-vascular remodelling is a mechanistic process delineated to the specific tissue type and specific stimuli. Therefore, with the exceptions of skeletal muscle responding to exercise and the female menstrual cycle itself, micro-vascular remodelling is limited solely to pathological situations, in particular inflammation, wound healing, ischemia, and that of hypoxia, together with a few other rare circumstances. Remodelling is mediated by monocytes and endothelial progenitor cells (EPCs) as shown in Fig.1 and is a mechanism that is dependent on changes in shear stress[25].
3.1. Tissue perfusion
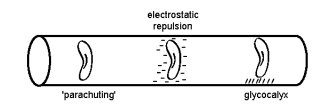
- Microvessels divide into numerous smaller branches over a given volume of tissue, thus maximising the available area for nutrient exchange. In this microenvironment, stasis of flow is prevented by the Fahraeus–Lindqvuist effect, repulsive charges between blood cells and vessel wall, as well as the thin glycocalyx film on the endothelial layer[5,7].
 | Figure 2. Factors preventing thrombogenicity in low-flow states[5] |
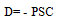 | (1) |
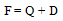 | (2) |
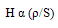 | (3) |
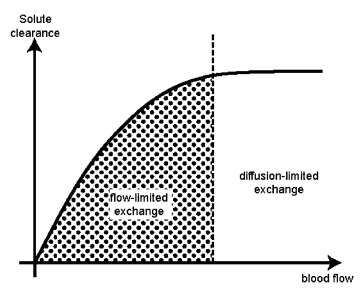 | Figure 3. Flow-and diffusion-limited exchange[5] |
3.2. Tissue-engineered microvessel beds: Micro-vascular tissue engineering
- A successful micro-vascular system depends on the graft material and how it is arranged. The material would have to be non-thrombogenic and must have similar compliance to native vessels to avoid intimal hyperplasia at its arterial end and must be sufficiently porous to allow nutrient exchange at the capillary level. An artificial capillary network would include small arteries (1–2 mm) conducting blood into an arteriolar network (100–1000 mm), which would eventually end in capillary-like vessels of 10–15 mm. These end capillaries would need to be within 150–200 mm of every target cell before converging into a venous collecting system. Therefore, both arteriolar and venular components of the scaffold would have to be in close proximity[6,8].
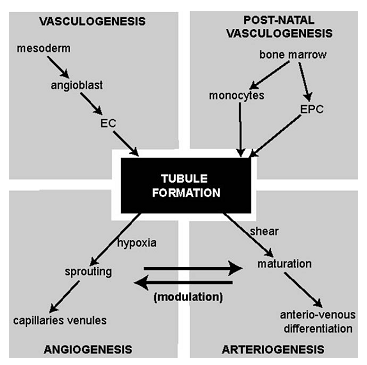 | Figure 4. The inter-relationships between various methods of vessel development. Keys: EC, endothelial cell; EPC endothelial progenitor cells[6] |
3.3. Types of prosthesis
- This depends on the triad of tissue formation: Microvessel networks, artificial matrices and neovascularisation constitute the triad of tissue vascularisation[1].
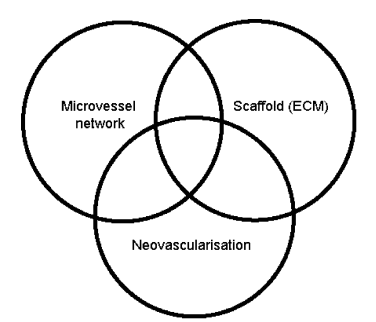 | Figure 5. Schematic diagram of cardiovascular prosthesis process[1] |
3.4. Macrovascular grafts
- The current results with PTFE and Dacron are clinically acceptable in peripheral bypass grafts, where as patency is significantly lower in grafts 6mm or smaller.
3.5. Micro-vascular grafts
- Micro-vascular grafts are those with internal diameters of 1mm or less, and may be classified into conducting arterial and distributing capillary vessels. The construction of arterial conduits is based on small-calibre vascular graft technology. These new grafts may be constructed by using (a) newer polymers such as CPU (b) by coating graft lumen with anti-platelet agents or cells[most commonly endothelial cells, but also possibly fibroblasts or smooth muscle cells (SMC)] and (c) constructing biological or bio-hybrid grafts in vitro prior to reimplantation. Although the autologous vein grafts[28], the current gold standard for micro-vascular repairs, are compliant and non-thrombogenic, they are limited by the need for additional vein-harvesting procedures. Furthermore, the construction of artificial vascularised tissue requires an inherent vascular network. Vein grafts are not suitable for this purpose as it is technically impossible to dissect out a capillary bed in its entirety.
4. Configuration of microvessels
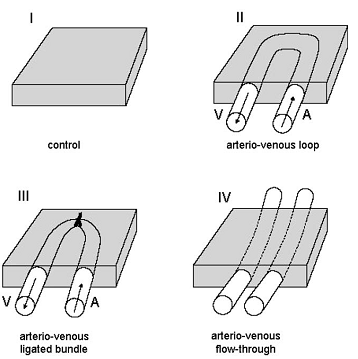
- The microvessels with a cross-sectional density of 1300 per mm2 and inter capillary distance of 34 μm to form a rich perfusing network. Tanaka and associates used the rat femoral vessel system to determine whether loop, ligated bundles or flow-through pedicles were most effective in initiating and sustaining tissue perfusion and neovascularisation[27]. The summary of that experiment can be shown as fig.6.
4.1. Biomaterial substitutes and extracellular matrix scaffolds (ECM)
- The ideal matrix should be biocompatible, porous, supportive, readily available, permeable to incoming vessels or chemicals, non-immunogenic, biodegradable, inert, have a slow-release mechanism and lastly be easily administered as an injectable. Tissue-engineered constructs act as temporary scaffolds, which maintain cells in situ and induce neovascularisation[1, 29].
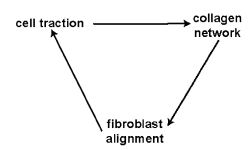 | Figure 7. The effect of cell traction on tissue patterns[29] |
4.2. Material used for cardiovascular tissue graft: Xenograft or vasculture
- Polyester, polyethylene terepthalate, Dacron, polytetrafluroethylene (PTFE), expanded polytetrafluroethylene (ePTFE) polyurethane etc. Poly (carbonate-urea) urethane (CPU) can also be used as the vascular graft with advanced effect of physiological shear stress[27-29].However, these prosthetic materials prove to be inferior to autologous conduits, especially when the vessel diameter is less than 5mm. The problems include increased risk of thrombosis and infection, limited durability, lack of compliance both of the graft and around the anastomosis, and failure due to restenosis, thus necessitating further interventions. Currently several groups are working towards the development of ‘living grafts’[30], seeded grafts and hybrid grafts.
4.3. Problem associated with cardiovascular (ECM) products
- i Increased risk of thrombosis and infection[1]ii Lack of compliance or potential to match both of the graft and around the anastomosis - Neointiminal hyperplasia[5]iii Sensing Inflammation[2,9]iv Failure due to restenosis, thus necessitating further inventionsv Growing of EC in an unorientated fashion by transanastomotic endotheliazation rather than excellent oriented mode of transmural or blood-borne endotheliazation[8]vi Shocking problem inside the patient body- due to bad implantation of arterio-venous valves[21-22]vii Failure due to occlusion inside the blood vessel due to abnormal obstructionviii Blood vessel hardening or elasticity problem during post-operational period: after 1-2 years[26]ix Biodegradability problem of implanted graft or ECM[1,7]
5. Probable solutions of previous cardiovascular problems
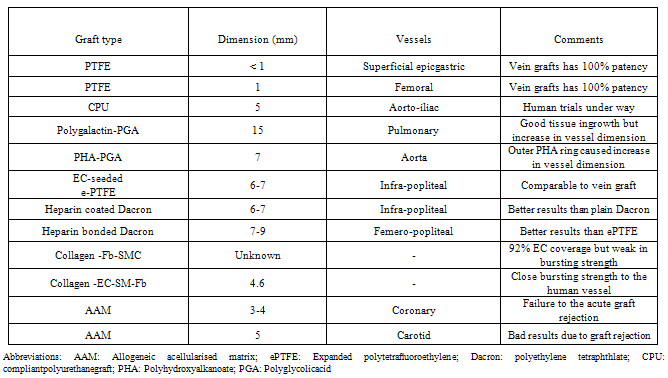
- There may be different ways by which a ideal and effective vascular graft can done as listed in table 2.
5.1. Graft made by synthetic protein polymers cross-linked by γ-rays- new generation biopolymers:
- Synthetic protein polymers cross-linked by γ- irradiation represent a new generation of biopolymers. Preliminary reports suggest that they have similar elasticity as arteries, with a controllable rate of degradation. This recent trend illustrates the limitations of PTFE and Dacron at lower flow rates and stresses the need for alternative biomaterials. Materials such as PTFE and Dacron have little potential for micro-vessel grafts due to their poor haemodynamics caused by lack of arterial compliance[10]. This results in thrombogenicity to the extent that in low-flow applications (as is the care for microvessels) below 4mm their usage is predelicted by their propriety for blockage. Polyurethanes such as CPU due to their superior haemodynamics (a result of their arterial like visco-elasticity and honeycomb structure) have high applicability for microvessel development and have shown little blockage in 2mm applications.These new grafts may be constructed by using (1) newer polymers such as CPU (2) by coating graft lumen with anti-platelet agents or cells[most commonly endothelial cells, but also possibly fibroblasts or smooth muscle cells(SMC)] and (3)constructing biological or bio-hybrid grafts in vitro prior to reimplantation[25,32]. Nylon can also be used for this type of polymer purpose-to provide good to moderate mechanical strength properties. The model has been shown as in the fig.8.
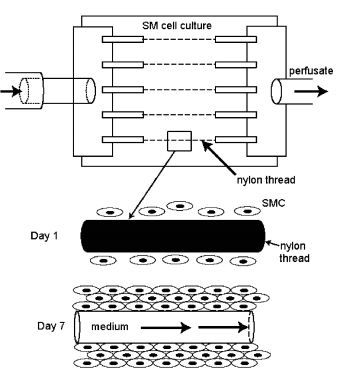 | Figure 8. Microvessel perfusion system[32] |
5.2. Sol-gel system
- Gels consist of three components namely- solute, solution, and voids (air swollen macromolecules of the polymers). Example: photo-polymerisable polyvinyl alcohol (PVA), triblock co-polymers of poly (L- lactide) / PLLA and poly (ethylene oxide) (PEO) (PLLA-PEO-PLLA). These types of polymer molecules are initially functionalised with adhesion factors. These are then cross-linked via the formation of reactive termini. Cells migrating into this matrix break down the cross-linking peptide leading to local degradation and hence release of the incorporated factors such as heparin and VEGF (vascular endothelium growth factors). These synthetic gels need to be bio-degradable and cell-responsive[21]. Using a PEG-based polymer by the shortening the block length from a mean molecular weight of 930 to 6090 kDa. A typical process of gel of polyethylene glycol (PEG) has been shown as in the fig.8.
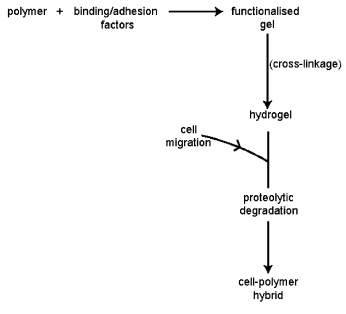 | Figure 9. The synthesis of biomimetic gels[21] |
5.3. Fibriller PU grafts
- With very rare exceptions fibrillar PU grafts are largely impenetrable for transmural tissue ingrowth. Only particularly large inter-fibrillar sp aces allow complete fibro-connective tissue penetration throughout the wall thickness. From day one the midgraft region is continuously covered by a fibrin layer hardly diminishing in thickness during the course of the first year. The fibrin layer itself is not as mighty as on knitted Dacron, sometimes giving a ‘ghost impression’ of the underlying prosthetic fibres. The outside capsule and scar formation is usually thin and dense with occasional FBGC wrapping around PU fibres .At2 weeks the FBGC become more prevalent increasing until week 6. The inflammatory reaction does not further increase between week 6 and 12[8, 10].
5.4. Foamy PU grafts
- In microporous PU foams with less than 15 μm pore size, there is relatively little ingrowth, even over an extended period of time. Below this cut-off point, only plasma-like insudation with some erythrocytes and a few clusters of white blood cells are found in the depth of the wall. Macrophages, giant cells and a few fibroblasts may penetrate a short distance into the implant if the pore size is borderline. By introducing these well- defined, equally sized spherical pores and large, equally well defined inter connectivity; we could determine the interdependence of inflammation and vascularisation, as well as the reproducible chronology of the tissue responses[33].
5.5. ePTFE grafts- the potential role of fibrin
- As we know that fibrin is the most important vascular endothelium growth factor (VEGF) of the blood vessels. So in this case we use a preteated heparin or fibrin seeded cells on an ePTFE or Dacron for a particular animal[10]. Ongoing platelet activation provides high concentrations of granule products like platelet factor 3, fibrinogen and von Willebrand factor as well as dense granule contents such as calcium. At the same time, graft surfaces are continually rinsed by blood with its undiminishing concentrations of fibrinogen and other clotting factors- like TCF-β (Thrombin Growth Factor)[26]. Thus, the fibrin generated on the inner surface of vascular prostheses has a particularly high level of fibrinogen – hence excellent healing properties.
6. The quality of a successful vascular graft
- i Be infection-resistant[1]ii Be biocompatible (non inflammatory, non toxic , non carcinogenic, non-immunogenic) and biostable[5,7]iii Be leak-proof and thromboresistant, but with adequate porosity for healing and angiogenesis. These attributes are usually provided within an artery by an intact endothelium, which also acts as a secretory tissue and selective permeability barrier[10].iv Have appropriate mechanical properties (strength, kink resistance, compliance, good suturability). The vessel must be strong enough to withstand considerable internal pressure before bursting, without kinking when in position. It must also allow sutures to hold under longitudinal and circumferential tension and retain axial and radial compliance and pulsatility[4].v Possess appropriate vasoactive physiological properties including the ability to constrict or relax in response to neural or chemical stimuli[5,10].vi Be able to be manufactured cheaply in a relatively short space of time, and in sufficient numbers with differing specifications (diameter, length, etc.) to meet commercial demand and fit the graft[2,9].
7. Conclusions
- Many of the experimental concepts for the vascular grafts of tomorrow promise to overcome the main obstacles plaguing those prostheses currently available for clinical implantation. At the same time, resolving one problem may introduce another. By eliminating the underlying cause of the chronic foreign body response by using resorbable materials, for example, the resulting scar tissue may deprive us of that very compliance which we tried to introduce through new elastomers. Similarly, by implanting dense, tissue-engineered tubes of smooth muscle cells, we may deprive our selves of the possibility of allowing transmural endothelialisation—should the engineered endothelium undergo the initial detachment often seen in veingrafts. However, as much as all these new problems will need to be addressed in a dynamic way as they come up, the overall questions asked and models used need to be of relevance. Moreover, understanding where we were misled in the past will be as helpful as understanding the obstacles which made half a century of SMG research so frustrating and unrewarding.
References
| [1] | P.Z.D. Bezuidenhout., and Human, P., 2007, Prosthetic vascular grafts: Wrong models, wrong questions and no healing., Biomaterials, 28(1), 5009 – 5027. |
| [2] | Kannan, R.Y., Salacinski, H.J., Sales, K., Butler, P., and Seifalian. A.M., 2005, The roles of tissue engineering and vascularisation in the development of micro-vascular networks: a Review., Biomaterials 26(1), 1857-1875. |
| [3] | Salacinski, H.J., Tai, N. R., Punshon, G., Giudiceandrea, A., Hamilton, G., and Seifalian. A.M., 2000, Optimal Endothelialisation of a New Compliant Poly(Carbonate Urea)Urethane Vascular Graft with Effect of Physiological Shear Stress Eur J. Vasc Endovasc Surg. 20, 342–352. |
| [4] | Rashid, S.T., Salacinski, H.J., Hamilton, G., and Seifalian. A.M., 2004, The use of animal models in developing the discipline of cardiovascular tissue engineering: a review, Biomaterials 25, 1627-1637. |
| [5] | Thomas, A.C., Campbell, G.R., Campbell, J.H., 2003, Advances in vascular tissue engineering, Cardiovascular Pathology 12, 271- 276. |
| [6] | Conklin, B.S., Richter, E.R., Kreutziger, K.L., Zhong, D.S., and Chen, C., 2002, Development and evaluation of a novel decellularized vascular xenograft, Medical Engineering & Physics 24, 173–183. |
| [7] | Crombez, M., Chevallier, P., Gaudreault, R.C., Petitcler, E., Mantovani, D., Laroche, G., 2005, Improving arterial prosthesis neo-endothelialization: Application of a proactive VEGF construct onto PTFE surfaces, Biomaterials 26, 7402–7409. |
| [8] | Guidoin, R., Sigot, M., King, M., and Luizard, M.F.S., 1992, Biocompatibility of the Vascugraft: evaluation of a novel polyester urethane vascular substitute by an organotypic culture technique, Biomaterials 13(5), 192-201. |
| [9] | Zhang, Z., King, M., Guidoin, R., Therrien, M.C., Doillon, W.L., Jonest, D., Huebner, E., 1994, In vitro exposure of a novel polyestetiethane graft to enzymes: a study of the biostability of the Vascugraft arterial prohesis, Biomaterials 15(14), 248-254. |
| [10] | Marois, Y., Guidoin, R., Boyer, D., Assayed, F., Doillon, C.J., Pasmter, R., and Marois, M., 1989, In vivo evaluation of hydrophobic and fibrillar microporous polyetherurethane urea graft, Biomaterials 10(1), 511-518. |
| [11] | Yeoman, M. S., Reddy, D., Bowles, H.C., Bezuidenhout, D., Zilla, P., and Franz, T., 2010, A constitutive model for the warp-weft coupled non-linear behavior of knitted biomedical textiles., Biomaterials, 31(32), 8484-8493. |
| [12] | Gundy, S., Manning, G., O’Connell, E., Ella, V., 2008, Harwoko, M.S., Rochev, Y., Smith, T., and Barron, V., Human coronary artery smooth muscle cell response to a novel PLA textile/fibrin gel composite scaffold., Acta Biomaterialia, 4(6), 1734-1744. |
| [13] | Wise, S.G., Byrom, M.J., Waterhouse, A., Bannon, P.G., Ng, and M. K.C., Weiss, A, 2011, A multilayered synthetic human elastin/polycaprolactone hybrid vascular graft with tailored mechanical properties., Acta Biomaterialia, 7(1), 295-303. |
| [14] | Jean, A., and Engelmayr, G.C., 2010, Finite element analysis of an accordion-like honeycomb scaffold for cardiac tissue engineering., Journal of Biomechanics, Volume 43(15), 3035-3043. |
| [15] | Szentivanyi, A., Chakradeo, T., Zernetsch, H., Glasmacher. B., 2011, Electrospun cellular microenvironments: Understanding controlled release and scaffold structure., Advanced Drug Delivery Reviews, 63(4-5), 209-220. |
| [16] | Yeoman, M. S., Reddy, D., Bowles, H.C., Bezuidenhout, D., Zilla, P., and Franz, T., 2010, A constitutive model for the warp-weft coupled non-linear behavior of knitted biomedical textiles., Biomaterials, 31(32), 8484-8493. |
| [17] | Jean, A., and Engelmayr, G.C., 2010, Finite element analysis of an accordion-like honeycomb scaffold for cardiac tissue engineering., Journal of Biomechanics, 43(15), 3035-3043. |
| [18] | Wise, S.G., Byrom, M.J., Waterhouse, A., Bannon, P.G., Ng, and M. K.C., Weiss, 2010, A multilayered synthetic human elastin/polycaprolactone hybrid vascular with tailored mechanical properties., Acta Biomaterialia, 7(1), 295-303. |
| [19] | Venkatraman, S., Boey, F., and Lao, L. L., 2008, Implanted cardiovascular polymers: Natural, synthetic and bio-inspired., Progress in Polymer Science, 33(9), 853-874. |
| [20] | Merwe, H.V., Reddy, B.D., Bezuidenhout, P.Z.D. and Franz, T., 2008, A computational study of knitted Nitinol meshes for their prospective use as external vein reinforcement., Journal of Biomechanics, Volume 41(6), 1302-1309. |
| [21] | McKenna, K.A., Hinds, M.T., Sarao, R.C., Wu, P., Maslen, C.L., Glanville, R.W., Babcock, D. and Gregory, K.W., 2012, Mechanical property characterization of electrospun recombinant human tropoelastin for vascular graft biomaterials., Acta Biomaterialia, 8(1), 225-233. |
| [22] | Baptiste, E.J., Blanchemain, N., Martel, B., Neut, C., Hildebrand, H.F., and Haulon, S., 2012, Safety, Healing, and Efficacy of vascular Prostheses Coated with Hydroxypropyl-β-cyclodextrin Polymer: Experimental In Vitro and Animal Studies., European Journal of Vascular and Endovascular Surgery., 43(2), 188-197. |
| [23] | Adamus, G., Sikorska, W., Janeczek, H., Kwiecien, M., Sobota, M. , and Kowalczuk, M., 2012, Novel block copolymers of atactic PHB with natural PHA for cardiovascular engineering: Synthesis and characterization., European Polymer Journal, 48(3), 621-631. |
| [24] | Peck, M., Dusserre, N., McAllister, T.N., and L'Heureux, N., 2011, Tissue engineering by self-assembly., Materials Today, 14(5), 218-224. |
| [25] | Koch, S., Flanagan, T. C., Sachweh, J.S., Tanios, F., Schnoering, H., Deichmann, T., Ella, V., Kellomaki, M., Gronloh, N., Gries, T., Tolba, R., Rode, T.S., and Jockenhoevel, S., 2010, Fibrin-polylactide-based tissue-engineered vascular graft in the arterial circulation., Biomaterials, Volume 31(17), 4731-4739. |
| [26] | Berreklouw, E., Leontyev, S., Ossmann, S., Velten, C., Vogel, B., Dhein, S., and Mohr, F.W., 2011, Sutureless mitral valve replacement with bioprostheses and Nitinol attachment rings: Feasibility in acute pig experiments.,The Journal of Thoracic and Cardiovascular Surgery, 142(2), 390-395. |
| [27] | Chakfe, N., Dieval, F., Wang, L., Thaveau, F., Rinckenbach, S., Tally, S.E., Mathieu, D., Magnen, J.F.L., Riepe, G., Kretz, J.G., and Durand, B., European Research Group on Grafts Used in Vascular Surgery (ERGGVS)., 2008, In Vitro Approach to the Dilative Behavior of Knitted Vascular Prosthetic Grafts., Annals of Vascular Surgery, 22(3), 402-411. |
| [28] | Chung, S., Ingle, N.P., Montero, G. A., Kim, S.H., and King, M.W., 2010, Bioresorbable elastomeric vascular tissue engineering scaffolds via melt spinning and electrospinning., Acta Biomaterialia, 6(6), 1958-1967. |
| [29] | Sommer, G., and Holzapfel, G.A., 2012, 3D constitutive modeling of the biaxial mechanical response of intact and layer-dissected human carotid arteries, Journal of the Mechanical Behavior of Biomedical Materials, 5(1), 116-128. |
| [30] | Mestres, G., Zarka, Z.A., Madrid, C. G., and Riambau, V., 2010, Early Abdominal Aortic Endografts: A Decade Follow-up Results., European Journal of Vascular and Endovascular Surgery, Volume 40, Issue 6, December 2010, Pages 722-728. |
| [31] | Stollwerck, P.L., Kozlowski, B., Sandmann, W., Grabitz, K., and Pfeiffer, T., 2011, Long-term dilatation of polyester and expanded polytetrafluoroethylene tube grafts after open repair of infrarenal abdominal aortic aneurysms., Journal of Vascular Surgery, 53(6), 1506-1513. |
| [32] | Meinel, A.J., Germershaus, O., Luhmann, T., Merkle, H.P., and Meinel, L., In Press, Accepted Manuscript, Available online 11 February 2012, Electrospun matrices for localized drug delivery: Current technologies and selected biomedical applications., European Journal of Pharmaceutics and Biopharmaceutics. |
| [33] | Caves, J. M., Kumar, V.A., Martinez, A.W., Kim, J., Ripberger, C.M., Haller, C.A., and Chaikof, E.L., 2010, The use of microfiber composites of elastin-like protein matrix reinforced with synthetic collagen in the design of vascular grafts, Biomaterials, 31(27), 7175-7182. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML