-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Biological Engineering
p-ISSN: 2163-1875 e-ISSN: 2163-1883
2011; 1(1): 6-10
doi: 10.5923/j.ijbe.20110101.02
A Review of the History and Role of UHMWPE as A Component in Total Joint Replacements
Mrinal K Musib
Department of Orthopaedic Surgery and Rehabilitation Medicine, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA
Correspondence to: Mrinal K Musib , Department of Orthopaedic Surgery and Rehabilitation Medicine, SUNY Downstate Medical Center, Brooklyn, NY 11203, USA.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Since its introduction as a bearing component for Total Joint Replacements (TJR) by Dr. Charnley in the early 1960’s, Ultra-high molecular weight polyethylene (UHMWPE) has become the gold standard to fabricate one of the articulating surfaces of total hip, total knee and total shoulder prostheses. More than a million TJR’s are performed every year and is a multi-billion dollar industry. In-spite-of the overwhelming success of this medical procedure, aseptic loosening as a result of wear limits its longevity to 15-20 years. This review article deals with the history of UHMWPE, its material properties that make it an ideal candidate for total joints, implant-component fabrication procedures and provides insights as to why some of the implants eventually fail. Alternate bearing components like Co-Cr and Ti alloys and ceramics are beyond the scope of this review.
Keywords: TJR, UHMWPE, Wear-Debris, Osteolysis
Cite this paper: Mrinal K Musib , "A Review of the History and Role of UHMWPE as A Component in Total Joint Replacements", International Journal of Biological Engineering, Vol. 1 No. 1, 2011, pp. 6-10. doi: 10.5923/j.ijbe.20110101.02.
Article Outline
1. Introduction
- The advent of UHMWPE as a material to manufacture parts of artificial TJR’s started approximately in the year 1962, when Sir John Charnley implanted the first hip prosthesis. Since then it has been the material of choice for the fabrication of one of the articulating surfaces of total joints. Despite the overwhelming success of this restorative procedure, wear of the components and resulting aseptic loosening remains the preemptive problem that limits the lifespan of the these implants from 15-20 years.
 | Figure 1. Total hip implant consisting of a metallic stem and head. |
 | Figure 2. Acetabular cup fabricated from UHMWPE comprises the articulating surface of a total artificial hip. |
2. Fabrication of UHMWPE Components
- UHMWPE is a polymer of ethylene and its molecular chain may contain as-many-as 400,000 carbon units. The molecular weight (MW) of UHMWPE is the MW of ethylene multiplied by the number of ethylene groups [(CH2=CH2⟹-(CH2-CH2)n] and may be between 2 and 6 million grams/per mole.
 | Figure 4. Fibrils (few tens of nanometers in width and few microns in length) connecting individual UHMWPE particles. |
 | Figure 5. Virgin UHMWPE powder. |
 | Figure 6. Acetabular and tibial components fabricated of UHMWPE. |
3. History of PE as an Implant Material
- The concept of total joint arthroplasty came about in the 1950’s when Sir John Charnley conducted a series of experiments to study the low coefficient of friction of the natural joint. In 1958, these experiments resulted in the development of low friction arthroplasty with polytetrafluoroethylene (PTFE) as the bearing material. Due to extensive wear and biocompatibility issues Dr. Charnley was looking for a replacement for PTFE. In about 1960, UHMWPE (RCH 1000) was introduced to Dr. Charnley who put it into himself to confirm that it may be a more suitable material for joint articulation than PTFE. In 1962 he used UHMWPE to construct the bearing surface and in 1968 gamma irradiated UHMWPE for the total joints. The following year total joints fabricated using UHMWPE sterilized with 2.5 Mrad were commercially manufactured. During the early 1970’s Zimmer manufactured carbon fiber reinforced UHMWPE and about that time alumina ceramic was used in Japan to fabricate the head of the implant which articulated against UHMWPE. Later during the 1980’s the UHMWPE was further modified as a bearing material for total joints and highly crosslinked UHMWPE was introduced in 1999 [ ].UHMWPE in knee was also introduced in the 1960’s and based on the necessity may be uni-, bi-, or tri- compartmental or other designs and thus the design aspects of total knee (TK) is more complex than hip implants. Over 300,000 TK’s are performed each year in US alone. Although a successful medical procedure, about 10% fail after 10 years primarily due to pitting, third body wear, delamination and abrasion. The mechanisms of wear varies with the design aspects of TK components that result in larger contact stresses in the patellofemoral and tibial components compared to the acetabular component and as such TKR are more complex to design and fabricate.
4. Sterilization and Oxidation Issues
- The components are then sterilized, primarily using any of the three methods: ethylene oxide gas, gas plasma treatment or gamma irradiation. Of these techniques, ethylene Oxide (EtO) and gas plasma are surface sterilization techniques. In ethylene oxide (EtO) usually 100% EtO is used. It is a lengthy process of about 40 hr and requires preconditioning/exposure/forced aeration. It usually involves no oxidation or crosslinking. This process may leave toxic residue. In gas plasma technique, Radiofrequency (RF) energy is used to generate plasma from vaporized hydrogen peroxide/paracetic acid. It is expected not to leave any toxic residue. It takes less time, about 4 hr with no lengthy aeration requirements and has no oxidation/crosslinking effects. The most common method of sterilizing of conventional UHMWPE is gamma sterilization. The gold standard dosage is about 2.5 - 4 Mrad from a cobalt-60 source 2,3. The high energy generated during gamma radiation breaks some of the C-bonds in the PE chain and generates free radicals. These free radicals can react in one of three ways: a) Recombination; b) Oxidative Chain Scission and c) Cross-linking. Each of these reactions has different effects on the molecular weight and mechanical properties of the UHMWPE. Chain scission decreases the molecular weight of the polyethylene which in turn results in the degradation of mechanical properties3-7. During shelf storage, UHMWPE components that are gamma sterilized in air permeable packaging undergo oxidative degradation, resulting in increase in density and crystallinity and subsequent loss in mechanical properties5,8. Some studies have also attributed extensive damage to the mechanical properties to long term shelf ageing9,10. Such products become more susceptible to delamination, fracture, decrease in molecular weight, creep deformation and fatigue strength and potential to undergo abrasive wear11,12. Based on the deleterious effects of oxygen on the longevity of the implant, most manufacturers now-a-days no longer perform gamma irradiation in air. Currently sterilization with gamma irradiation is mostly done in an inert atmosphere. Sterilization of UHMWPE components under vacuum or in an inert oxygen free environment in presence of nitrogen or argon is also widely used. Sterilization in an oxygen free environment decreases oxidation whereas it facilitates recombination and cross-linking that decreases wear of the components in vivo. To prevent oxidation during radiation sterilization or during shelf life, an antioxidant; vit. E or its synthetic derivative alpha-tocopherol is being used13,14. Studies to understand the long term effects of inclusion of such additives to the biomechanical properties and biocompatibility of UHMWPE inserts are presently being conducted.
5. Crosslinking of UHMWPE
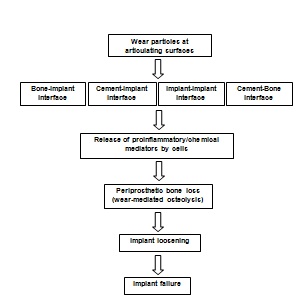
- Presently UHMWPE components are being crosslinked (linking of multiple molecular chains by covalent bonds) by high energy radiation. Although crosslinked UHMWPE may have better wear properties, they may have compromised mechanical characteristics compared to conventional PE. Crosslinking is primarily accomplished by using high energy radiation over 60 kGy and then annealing just below or above the melting point of UHMWPE (about 135℃) to quench the free radicals3. Crosslinking improves the wear characteristics and as such less number and volume of wear particles are generated which has been implicated in wear-mediated osteolysis. This has improved biocompatibility of crosslinked UHMWPE inserts.Crosslinking also decreases the number of wear debris that are generated at the articulating surfaces. As these debris have been implicated in osteolysis15-18, generation of less debris may theoretically increase the longevity of implant components. Studies have been conducted to isolate, fractionate and characterize these debris19. Previously chemical and enzymatic techniques have been used to isolate wear debris particles20,21. The results may help us better understand the phenomenon of wear-mediated osteolysis.
6. Conclusions
- Since its introduction into the field of orthopedics by Dr. Charnley over 50 years ago, UHMWPE is still the gold standard as an articulating surface for total joints. Oxidation as a result of high energy radiation and extended shelf life may cause wear-mediated osteolysis has been attributed to long term failure of total prostheses. Although much work still needs to be done to understand the underlying mechanism of its pathogenesis, it is generally accepted that periprosthetic bone resorption is initiated by an aseptic inflammation to wear debris generated from continuous wear, abrasion, or corrosion of implant components at the articulating surface. If left untreated, progressive osteolysis caused by wear particles can result in substantial bone loss and subsequent implant failure. To prevent oxidation and improve wear properties, UHMWPE components are being crosslinked and additives (antioxidant as Vit E. or its synthetic derivatives) are being included. This will help manufacturers to enhance the longevity of such inserts and thus prevent their premature failure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML