-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2025; 13(1): 1-8
doi:10.5923/j.ijbcs.20251301.01
Received: Dec. 30, 2024; Accepted: Jan. 20, 2025; Published: Feb. 13, 2025

Postoperative Cognitive Dysfunction After Major Orthopedic Surgery: Regional vs General Anesthesia
Visolatkhon Sharipova, Abror Valihanov, Azamat Alimov
Department of Anesthesiology and Intensive Care, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan
Correspondence to: Azamat Alimov, Department of Anesthesiology and Intensive Care, Republican Research Centre of Emergency Medicine, Tashkent, Uzbekistan.
| Email: |  |
Copyright © 2025 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Postoperative cognitive dysfunction (POCD) is associated with poor postoperative outcomes and these patients are at greater mortality risk compared to patients without neurocognitive decline. Numerous risk factors have been implicated in the development of POCD such as older age, lower level of education, preoperative cognitive impairment or delirium, type and invasiveness of surgery, depth of anesthesia, perioperative pain etc. Our objective was to compare the incidence of POCD after major orthopedic surgery receiving either general or regional anesthesia alone. Methods: A prospective, single centre, observational study included 112 patients undergoing major orthopedic surgery. Patients were allocated to either regional or general anesthesia groups. The perioperative neuropsychological testing included preoperative and two postoperative (7 days and 3 months after surgery) testing. Results: The incidence of POCD at 1 week after general and regional anesthesia was 9/62 (14.5%) vs 8/50 (16%), P=0.83. In 5 patients from each of the groups (8% vs 5%) delayed POCD after 3 months were diagnosed, P=0.52. Pain scores and postoperative complications did not differ significantly between groups. Conclusion: No significant difference was found in the incidence of cognitive dysfunction 3 months after either general or regional anesthesia.
Keywords: Major orthopedic surgery, Neuropsychological testing, Perioperative neurocognitive disorders, Postoperative cognitive dysfunction
Cite this paper: Visolatkhon Sharipova, Abror Valihanov, Azamat Alimov, Postoperative Cognitive Dysfunction After Major Orthopedic Surgery: Regional vs General Anesthesia, International Journal of Brain and Cognitive Sciences, Vol. 13 No. 1, 2025, pp. 1-8. doi: 10.5923/j.ijbcs.20251301.01.
Article Outline
1. Introduction
- Postoperative cognitive dysfunction (POCD), a condition that has been poorly recognized and defined for decades, is one of the most common postoperative complications, especially for the elderly [1].POCD is associated with poor postoperative outcomes. Patients classified as having POCD at the time of hospital discharge after noncardiac surgery are at greater risk of dying within 3 months than those without it, and if they have POCD at both hospital discharge and 3 months postoperatively, are nearly 5 times more likely to die 1 year later [2,3]. According to different authors, the incidence of POCD varies from 10% to 45% [4]. Numerous risk factors have been implicated in the development of POCD such as older age, lower level of education, frailty, cerebrovascular disease, type and invasiveness of surgery, repeat procedures, depth of anesthesia, perioperative pain etc. Advanced age is the best-established risk factor [5-7]. Low educational achievement is associated with greater risk and higher educational achievement is protective and this is a component of “cognitive reserve” [8].General anesthesia intuitively seems to produce long-lasting changes in cognition. Numerous preclinical data support this statement [9-12]. However, the clinical data are much less clear and a few studies which directly compared general anesthesia to regional anesthesia have found little or no difference in POCD [13,14].Newly proposed consensus recommendations suggest the term perioperative neurocognitive disorders (PND) which is any new cognitive dysfunction identified in the postoperative period. This includes delayed neurocognitive recovery (dNCR) when the decline occurs acutely postoperatively (including delirium) but persists for up to 30 days after surgery and neurocognitive disorder (NCD) when it lasts up to 12 months [15,16]. Taking into account the novelty of this nomenclature, in this paper we used the term POCD for clarity. The objective of this study was to compare the incidence of POCD after a major orthopedic surgery receiving either general or regional anesthesia alone.
2. Methods
- Clinical materialA prospective, single centre, observational study was conducted at the Republican Research Centre of Emergency Medicine (RRCEM), Tashkent, Uzbekistan. The study was approved by the Institutional Review Board. 123 patients aged 30 to 80 undergoing unilateral major orthopedic surgery were enrolled prospectively from November 2018 to June 2019. They were allocated either to regional anesthesia (spinal, epidural or peripheral nerve block) or general anesthesia group.Exclusion criteria were those who could not read or write; color-blind people; patients with hearing problems, those whose native language was not Uzbek nor Russian; patients with combined traumatic brain injury and trauma of the dominant upper limb; those with a history of psychiatric or neurological disorders (history of stroke, transient ischemic attacks, seizures, generalized anxiety, alcoholism or drug addiction); American Society of Anesthesiologists (ASA) physical status score > III; moderate and severe anemia (hemoglobin less than 90 g/l); severe intraoperative bleeding; those who had already undergone neurosurgical or cardiac procedures; those who refused to participate in the study. To identify and exclude patients with asymptomatic preoperative cognitive impairment The Short Test of Mental Status (STMS) was used as a screening method [17]. The maximum score of the method is 38 and those who did not obtain a minimum standard score of 29 points were excluded.
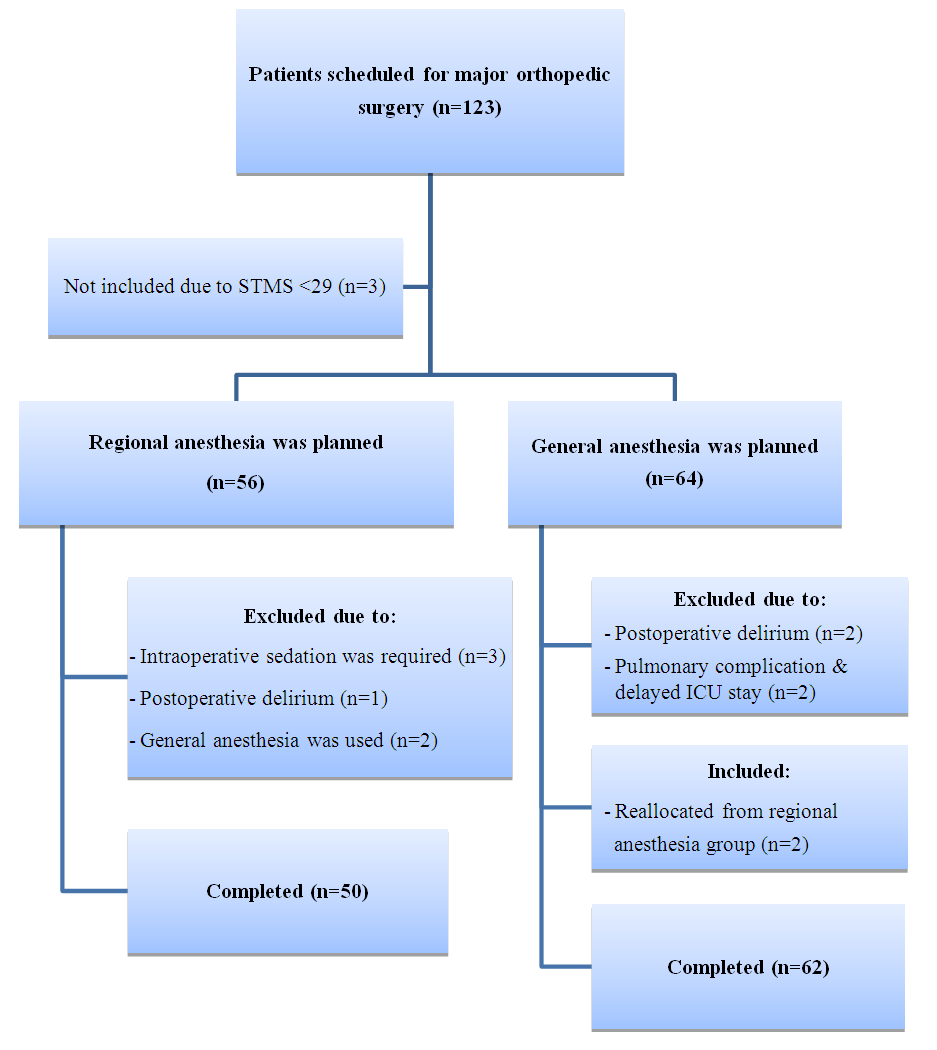 | Figure 1. Flow chart of patient enrollment |
3. Results
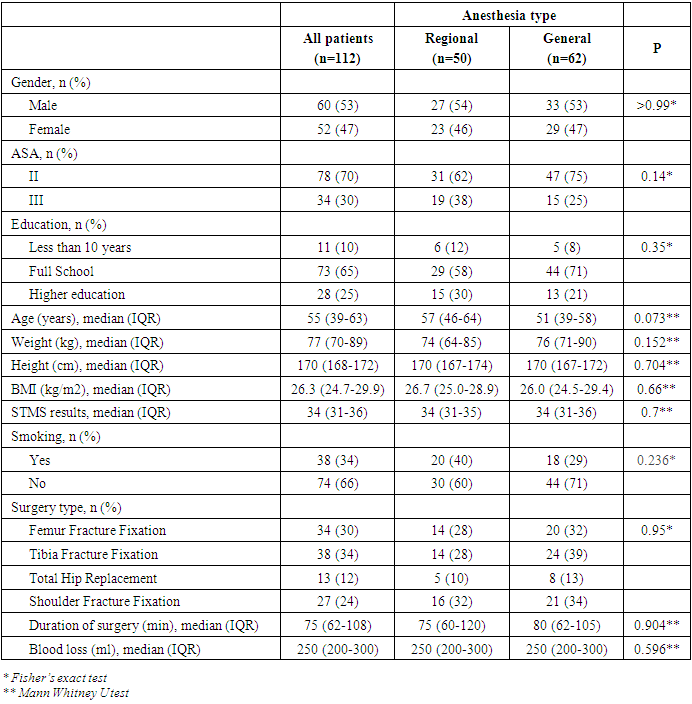
- Patient characteristicsAfter exclusion of 11 patients, the remaining 112 patients were eligible to participate in the study. Based on the type of anesthesia, patients were allocated into two groups: regional (n=50) and general (n=62) anesthesia groups. Regional anesthesia was unsuccessful in 2 patients allocated to regional anesthesia in whom general anesthesia was therefore necessary. Clinical, demographic and procedure data of patients are summarized in Table 1.
|
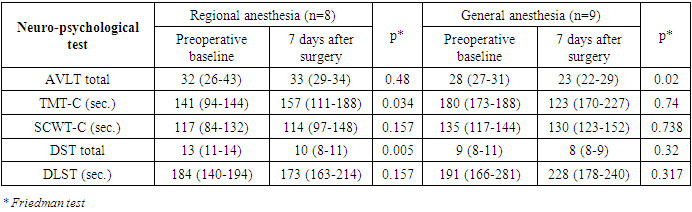
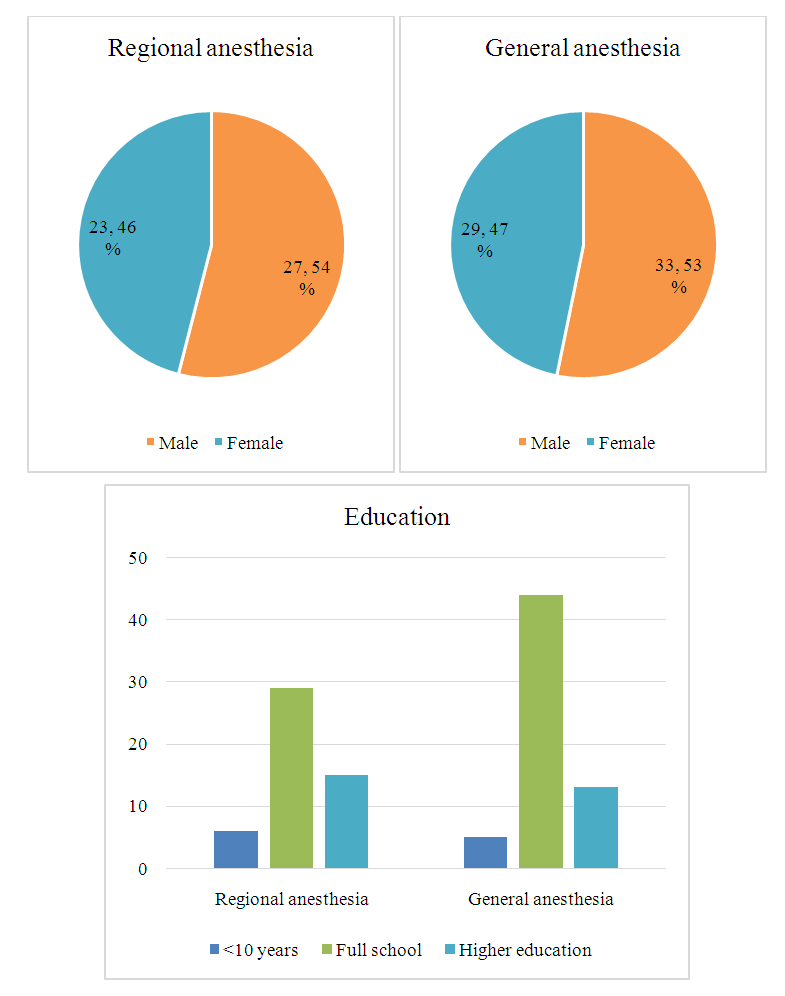 | Figure 2 |
|
|
|
|
4. Discussion
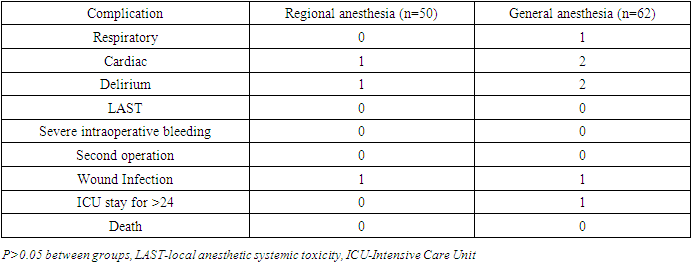
- In our study postoperative cognitive dysfunction was detected in about 15% of patients, at 1 week with a cognitive recovery in some patients after 3 months with no significant difference between general and regional anesthesia. Major limitations of the study are the relatively small number of patients and that it is a single-centre study. Taking into account the estimated incidence of POCD, the statistical power of the study would be not as high as we wished to be. However, the study included young and middle-aged patients while most research on this issue is performed in elderly. Although most patients who developed POCD in this study are older people our results showed that young age is not an exception to be involved by postoperative cognitive decline.The etiology of POCD is likely to be multifactorial. The effect of general anesthesia to cognitive function has been studied by many neuroscientists and clinicians in laboratory experiments as well as in clinical material. Xie et al. reported that exposure to isoflurane at 1.4% for 2 h increases caspase 3 and promotes the accumulation of amyloid beta which prevents new protein synthesis and one of the most evident mechanisms underlying Alzheimer disease activity in mice [23]. Saab et al. reported that an exposure to isoflurane of only 1 h impairs short-term memory [24]. Stratmann et al. reported decreased proliferation of progenitor cells in the hippocampal dentate gyrus in the P7 rat brain exposed to isoflurane, which lasted for at least 5 days, suggesting that this might be an important mechanism [25]. The neurotoxicity of anesthetic drugs has been studied by the same author in order to determine whether anesthesia in childhood might lead to cognitive impairment in later years. Studies have failed to yield any definitive evidence that anesthetic drugs are neurotoxic and findings are currently debated [26]. Earlier studies suggested an association between general anesthesia and a higher incidence of cognitive dysfunction relative to epidural anesthesia [27]. However, recent studies concluded that there was no relationship between anesthetic techniques and the magnitude or pattern of postoperative cognitive dysfunction [13,28]. Concerns that general anaesthesia would be susceptible to significantly contribute to POCD are not supported by the evidence from randomized controlled trials [29,30,31].Our observation did not show the direct influence of anesthesia type on cognitive function and early cognitive impairment in the general and regional anesthesia groups could be the result of negative effects of the general anesthetic agents or surgical stress induced neuroinflammation.In conclusion, we found no significant difference in the incidence of cognitive dysfunction 3 months after either general or regional anesthesia. Accordingly, there is no evidence to suggest any causative relationship between general anesthesia and long-term POCD and large, multicenter studies would help to clarify the role of etiologic factors in the development of perioperative neurocognitive disorders. When several options exist, we suggest that the choice of anesthesia type should be based on a discussion of patients’ preference, possible postoperative complications, and the experience of the anesthesiologists and anesthesia team.
ACKNOWLEDGEMENTS
- This work is supported by the Ministry of Health and Ministry of Innovative Development of the Republic of Uzbekistan (grant number FZ-2016-0907071434).
Institutional Review Board Statement
- The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Republican Research Centre of Emergency Medicine.Competing interests: None.
Authors’ Contributions
- VSH conceived of study design and critically reviewed the manuscript. AVA made substantial contributions to data collection, analyzed and interpreted participant data. AAH performed neuropsychological testing and was the primary author of the manuscript. All authors read and approved the final manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML