-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2018; 7(2): 31-35
doi:10.5923/j.ijbcs.20180702.01

Anatomical Investigation into the Brain of Wild African Parrot (Poicephalus senegalus versteri): Gross and Quantitative Study
Wanmi N.1, Ibe C. Samuel2, Sani S. Abdullahi3
1Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Benue State, Nigeria
2Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Michael Okpara university of Agriculture, Umudike, Abia State, Nigeria
3Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Usman Danfodio University, Sokoto State, Nigeria
Correspondence to: Wanmi N., Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Benue State, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Knowledge on the anatomy of the brain of parrot is important with respect to the current advancement in comparative avian anatomy. This investigation was aim at reporting some landmarks anatomical features on the brain of the wild African parrot that will be valuable in obtaining a baseline information in this specie. Ten apparently healthy adult wild African parrots were euthanized using Pentobarbital sodium (Nembutal®) at 40mg per body weight. Sex was not kept into consideration in this study. Quantitative study of the body and brain indicated mean weights to be 163 ± 4.36 g and 4.78 ± 0.21 g, with whole brain having length and width measuring 3.66 ± 0.19 cm and 2.06 ± 0.07 cm respectively. The cerebral surface is thrown into three deep depressions; the interhemispheric fissure flank by two cerebral vallecula. Two large bulges, the Wulst are prominent on the dorsum of the cerebral hemispheres an indication of a good cognitive behaviour. Extensively large optic tracts projects from optic lobes suggesting that the parrot possesses high auditory activity.
Keywords: Anatomical, Brain, Morphology, Parrot, Wild
Cite this paper: Wanmi N., Ibe C. Samuel, Sani S. Abdullahi, Anatomical Investigation into the Brain of Wild African Parrot (Poicephalus senegalus versteri): Gross and Quantitative Study, International Journal of Brain and Cognitive Sciences, Vol. 7 No. 2, 2018, pp. 31-35. doi: 10.5923/j.ijbcs.20180702.01.
Article Outline
1. Introduction
- The African parrot is of the Kingdom; Animalia, Phylum; Chordata, Class; Aves and Order; Psittaciformes. The Psittacus and Poicephalus are dominant in West Africa, often considered as similar taxa [1, 2]. Wild parrots has features, which includes; a strong, curved bill, an upright stance, strong legs, and claw zygodactyl feet. Most of the wild species possess diverse colour, exhibit little or no sexual dimorphism in the visual spectrum [3]. They form the most variably sized bird order in terms of body length. In the wild, their diets include seeds, nuts and fruits but sometimes eat animals and carrion [4]. Recently in Nigeria, most private schools have been involved in keeping pet animals of various species including parrots, which now make the demand for this bird to be on an increase because of its ornamental peculiarity. Despite it wide distribution, large scale domestication of this specie and its population has not been documented in Nigeria unlike that of the guinea fowl where large scale domestication has been established and its population has been estimated at 44 million in captivity [5]. Meat from parrots is yet to be considered as delicacy in Nigeria but are seen sold in cages in local market/road sides and this serve as source of income for rural dweller [6].Brain morphology using quantified data is an important area of research in neuroscience. Scanty documentation exist on the anatomy of the brain of parrot, especially in Nigeria. Work done on the anatomy of the wild parrot include; those on some body organs of the wild parrots [7]. This present study was aimed at investigating the gross and morphometric aspects of the brain of wild indigenous parrot and to correlates its behavioural pattern to live birds. Baseline data generated will aid in giving further information on brain of the wild Africa parrots.
2. Materials and Methods
2.1. Experimental Birds
- In this study, sex was not kept into consideration. Ten (10) apparently healthy wild adult African parrots were purchased from a village marketer in Shika, a village outskirt of Zaria town, Kaduna State Nigeria. Birds were transported in a standard ventilated Laboratory cage to the animal unit in the Department of Veterinary Anatomy, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. They were kept and fed for a period of one month for pre-conditioning using ground-nut, watermelon and water given ad libitum.
2.2. Animal Handling, Restraint and Treatment
- Parrots when calm are friendly but aggressive when not properly handle. Rope made of cotton was used to restrain the parrots. This was achieved by gently holding the base of the wing while another hand placed on the feet of the parrot. By this, the parrot is lifted out of the cage and place on the surface of the table. At this point the legs and wings are pull towards the body and a rope is tied round the wings, legs and body. Each live bird was weighed using digital electronic balance (Citizen Electronic Scale, PVT. Ltd. Hamburg, Germany, Sensitivity: 0.01g), weight of rope was taking before the restrain was made. When the parrots were weight, they were euthanize using (Pentobarbital sodium) Nembutal® at 40 mg per body weight before brain extraction.
2.3. Brain Extraction
- Extraction of brain was achieved by making an incision from the left dorsal orbital rim to the right dorsal rim of the opposite side over the frontal bone with a surgical blade; another cut was made from the lateral canthus to the point of the nostrils on both sides. This was repeated using a hand saw-like blade for bony structures, with a gentle traction the forebrain was exposed. To expose the cerebellum, a separate incision was made along the lateral canthus of the eye on either side over the foramen magnum. The falx cerebri and tentorium cerebellar were separated thereby exposing the brain in situ. The cranial nerves and meninges were carefully severed as the forebrain is elevated from the skull floor; the brain was removed as described by [8] and immersed in normal saline while morphologic evaluations progresses.
2.4. Brain Morphometry and Morphology
- Immediately after each brain was extracted, they were weighed using (sensitive electronic balance; Mettler P 1210, AG, Switzerland, Sensitivity 0.001g). Length and width were obtained using ruler and digital Vernier caliper MG6001DC (General Tools and Instruments Co., New York; sensitivity of 0.01 cm) with values recorded in centimeter. Definitions of gross anatomical structures was based on standard information on avian anatomy, Nomina Anatomica Avium [9].
2.5. Separation of the Various Brain Components
- The forebrain was separated from the rest of the brain by exposing the cerebral crura at the caudal poles of the cerebrum. A cut was made at this point and the forebrain detached. Once the forebrain is removed, the brain stem and the cerebellum is exposed. The left and right cerebral hemispheres were separated by cutting through along the interhemispheric fissure and corpus callosum using a scalpel blade. The cerebellum is attached to the brain stem via two cerebellar peduncles. Incision was made at the base of the lateral peduncles (brachium restiformis and brachium conjunctiva) to remove the cerebellum. The incision into regions of pontimesencephalic and pontimedulary flexures was made to free midbrain, pons and medulla oblongata from the brainstem.
2.6. Statistical Analysis
- Analysis of the body, brain and its component was made using statistical package for social science (SPSS) version 17.0. In the analysis, the descriptive statistics was expressed as Mean ± Standard Error of the Mean. Student t-test was used to compare the level of significant and value of p < 0.05 was considered significant.
3. Results
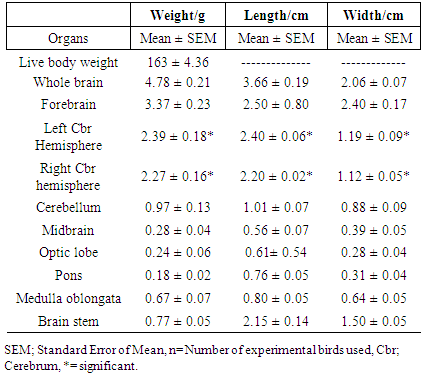
3.1. Brain Morphometry
- The mean body and brain weights of parrot were 163 ± 4036 g and 4.78 ± 0.21 g respectively. The mean length and width of the brain were 3.66 ± 0.19 cm and 2.40 ± 0.07 cm respectively. The mean length and width of the forebrain were almost same size but not statistically significant. The mean weights, length and width of the left and right cerebral hemispheres were significant at p < 0.05. The mean weight and length of the cerebellum was 0.97 ± 0.13 g and 1.01 ± 0.07 cm. The midbrain and optic lobes are derivatives of the mesencephalon in birds with mean weights to be 0.28 ± 0.04 g and 0.24 ± 0.06 g respectively. The brainstem had a mean weight, length and width to be 0.77 ± 0.05 g, 2.15 ± 0.14 cm and 1.50 ± 0.05 cm respectively.
3.2. Gross Morphologic Features
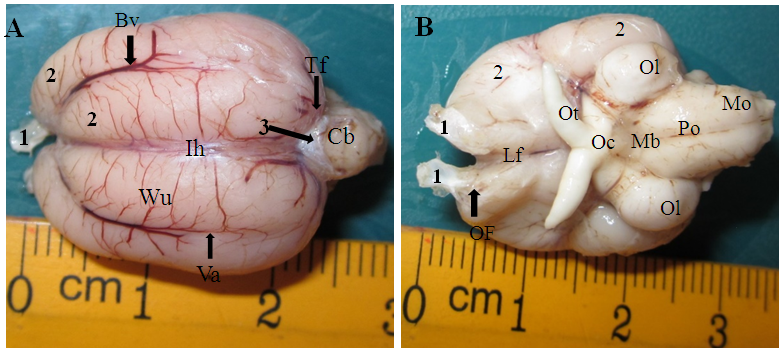
- The dorsal cerebral surface of the brain was observed to be thrown into three depressions that meet at the rostral inception of the interhemispheric fissure that appears as curbs. The right and left cerebral vallecula extends caudally from the rostral limit of the interhemispheric fissure which is deeper and gradually faint out at the caudal pole of the cerebral hemispheres. The vallecula merges with Wulst at the caudal pole to form a convex cerebral surface. The vallecula is markedly occupied by large blood vessels which tappers out caudally. The right and left Wulst is the most swollen part on the dorsum of the cerebrum. It is partition by an interhemispheric fissure medially and laterally it is bounded by right and left vallecula. It narrows rostrally and broaden caudally with blood vessels crossing its surfaces. The dorsal cerebral surfaces was seen to be tremendously marked by vallecula, interhemispheric fissure, Wulst and the olfactory bulb. The cerebellum is relatively smaller, projected upward and separated from the caudal limits of the cerebral hemispheres by thick arachnoid fold (Figure 1A). The olfactory bulb was seen attach to the rostral limits of the cerebral hemispheres by an olfactory stalk. The optic tracts are large whitish stalk-like exiting from the optic lobes that bifurcates to appear as two cornual processes. The right and left optic lobes appears round to oval like projections on the caudoventral ends of the cerebrum (Figure 1B).
4. Discussion
- The mean brain weight have been reported to be lower than body weight of most species of bird and could thus differ in birds of the same body weight [10]. The lengthening nature of the brain might be as a result of the forward protruded skull and elongation of the cervical region which might have influence brain length and weight [11]. The left cerebral hemisphere was slightly higher than the right side and was significant at p< 0.05. This agree with the general statement that functional asymmetry of the brain can be used in testing hemispheric variation in the brain of birds which have great effects on the functionality of an organ [12, 13] and side of the body which the brain control [14]. This implies that the left cerebral hemisphere in the wild Africa parrot has greater activity over the right cerebral hemisphere on the part of body it control. Presence of large olfactory bulb show that the parrot possesses a relative sense of olfaction. Olfaction in birds in the wild is for survival from predators and location of prey and birds possesses variable degree of olfaction and this is similar with the general report that the olfactory sense of birds is poor [16] and could differ in some species of bird such as the brown Kiwi, vultures and canaries which have well-developed sense of smell [17, 18]. The vallecula in birds aid in visual acuity and is slightly convex, curved supersulcus bending posterior-medially on either sides of the cerebrum. This statement is in agreement with those of [19] in Africa ostrich, but disagree with those [20] in domestic pigeon where they are found on the lateral surface of the cerebral hemisphere. These morphological features could be useful in specie differentiation and birds which vallecula are place superiorly has good visual acuity. This indication makes parrot to have good visual to locate it prey in the wild. The Wulst or the hyperpallium is located on the anterior dorsal portion of the cerebral hemispheres. This is similar to report of Stingelin, that Wulst in birds correspond to mammalian cerebral complex cognitive functions that are associated with higher-level consciousness in mammals [21]. The Wulst is place anterior-most on the cerebrum in helmeted guinea fowl [22] and posterior-dorsal in duck [23]. The domestic pigeon showed a well-developed Wulst, suggesting that the level of consciousness is high and is an important sensory organ in the pigeon.The cerebellum in the wild Africa parrot is relatively not folded. Folding of the cerebellum influences motor behaviour, eye movement, balance with display of different forms of flight, dexterity and posture [24]. This implies that the wild Africa parrot has to stabilize is motor system before taking on its prey. The cerebellum was also observed to be small, rounded to oval which is similar to those of the chicken and pigeon [25]. The size and position of the cerebellum in the band owl, vultures and eagle is round, positioned upward and backward [26]. These variations explain the fact that size and position of the cerebellum in birds is associated with size and posture of the bird and most large birds possess smaller size of cerebellum and are mostly flightless or short distance flyers [27]. The shape of the optic lobe is relatively small and oval-like structure. This finding is in agreement with reports on kiwi, band owl and humming birds [28], but disagree with reports on pigeon where they are large and circular in shape [29]. Generally, parrots are said to possess a very small optic lobe [30].
5. Conclusions
- Presence of olfactory bulb indicates good olfaction in the parrots. The cerebellum with less folding show parrots are short distance fliers with less dexterity. Research in neuroscience is using various tools, considering the fact that there is growing concern over adaptation from the wild to domestication in birds.
ACKNOWLEDGEMENTS
- We sincerely thank all staff of the animal house and gross Anatomy Laboratory in the Department of Veterinary Anatomy, A.B.U Zaria for their immense supports.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML