-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2017; 6(3): 58-64
doi:10.5923/j.ijbcs.20170603.03

Cerebellar Cortex Morphology of the Red Sokoto Goat (Capri Hircus): Foliation Pattern
Byanet O. , Joshua D. Mkaanem
Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture
Correspondence to: Byanet O. , Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Morphological analysis of the cerebellar cortex was done on the red Sokoto goats, with the aim of providing reference baseline data in this species. Results from the nine cerebella examined revealed the mean cerebellar weight of 10.2 ± 0.38g, this accounted for 14% of the total brain weight. The cerebellar mean length 4.9 ± 0.13cm, was close to its width 4.4 ± 0.16cm were very close. The cerebellar surface was complexly foliated, with a well-developed central vermis the cerebellar hemispheres and paraflocculus. The lingula, central lobule and culmen were placed cranioventrally and were not visible on the dorsal view. The declive was the largest lobule in the rostral lobe, having five sublobules. The broader lobules in the caudal lobe were Folium vermis and Tuber vermis, each had two parts; (lobule VIA, VIB) and (lobules VIIA, VIIB), respectively with their sublobules. Furthermore, lobule IX was the third large lobules in this lobe with four sublobules (IXa - IXd). In conclusion, the relatively small sizes of lingula, central and culmen lobules may be correlated with the characteristic short and erect tail of the animal. In addition, the well-developed bilateral cerebellar hemispheres and paraflocculus in red Sokoto goat may be related to the animal high motor activities such as climbing, standing erect on the hindlimbs, with the forelimb suspended in the air to grasps leaves of short trees and shrubs in its ecological regions.
Keywords: Cerebellum, Lobe, Lobule, Foliations, Red Sokoto goat
Cite this paper: Byanet O. , Joshua D. Mkaanem , Cerebellar Cortex Morphology of the Red Sokoto Goat (Capri Hircus): Foliation Pattern, International Journal of Brain and Cognitive Sciences, Vol. 6 No. 3, 2017, pp. 58-64. doi: 10.5923/j.ijbcs.20170603.03.
Article Outline
1. Introduction
- In northern Nigeria, small ruminant farming occupies an important place in the economy of many rural household. The three major recognized breeds of goat in Nigeria are; the West African dwarf, the red Sokoto and the Sahel goats [1], with estimated population of 16.28 million for red Sokoto goats, 14.62 million for West African dwarf goats, and 1.62 million for Sahel goats in Nigeria. The Red Sokoto goat is probably the most widespread and well known type in Nigeria [2], are found in Sokoto and Kano states. Apart from its meat, the red Sokoto goat is the source of leather used for production of shoes, belts, and bags. The red Sokoto goats are relatively small size (male, 60 to 65cm; female, 54 to 65cm, in height); both sexes have horns, though slightly heavier in males. The horns are flattened dorsoventrally and grow backwards toward the head and neck, with narrow intercornual space [3]. The neck is short but seen to be very mobile. Ears are short, medium width size, and carried horizontally. The limbs are short, but strong with well-developed muscles, which enable the animal to stand upright. Tail is usually short; stands erect and covered with fine and short hairs, with males having longer waiver hairs. The red colour from which it earned its name, distinguished the red Sokoto goat from other goats [3], and are said to have certain characteristics such as maintenance of visual contact and associating in groups similar to other mammals. It has been observed that relative brain and body sizes present an excellent example of two highly positively correlated traits across various taxonomic levels [4]. Cerebellum as part of nervous system varies in relative size and its folding ability in domestic animals [5]. It is important in the control and coordination of movements, behavioral emotion, posture and cognition in man and animals (Fletcher, 2006) [6]. The nervous system has been incompletely studied, with little information on morphometric and brain surface descriptions [7]. Detail gross morphology of the cerebellum with its longitudinal divisions and lobulation pattern in the red Sokoto goat is lacking. This work was aimed at analyzing the gross morphological structure of the cerebellar cortex in red Sokoto goat to provide baseline reference data. The basic structural knowledge of the morphology of this organ will prove of value in future experimental researches and may also help in interpreting some behavioral adaptation of these animals in their ecological zones.
2. Materials and Methods
2.1. Animal Source and Study Location
- A total of nine (9) heads of Nigerian indigenous breeds of red Sokoto goats were used in this study. Some of these heads were purchased in Makurdi metropolis abattoir and transport in buckets containing cold pack [8] to the Laboratory, Department of Veterinary Anatomy, University of Agriculture, Makurdi, where the research was conducted.
2.2. Extraction of Cerebellum
- The head weights and dimensions were taken before skinned and stripped of all muscles. After a week, the brains were then extracted from the skull as described for ruminants, [6] and fixed in 10% formalin. The extracted brains were generally observed for gross pathological lesions. When proved normal, they were then weighed with a mettler balance, (Model P 1210, AG, Switzerland). The cerebellum was separated from the brain stem by cutting through the cerebellar peduncles using a surgical blade. The cerebellar weight and dimensions were obtained. The terminologies used were adopted [9, 10] and the 5th Edition (revised version) [11].
3. Results
3.1. Morphometry
- The mean weights of the head, brain and cerebellum were 1377.8 ± 46.48 g, 72.3 ± 0.98 g and 10.2 ± 0.38 g, respectively. The cerebellum weight therefore accounted for 14% of the total brain weight. The cerebellum length (4.9 ± 0.13cm) was slightly longer than its width (4.4 ± 0.16cm) (Table 1).
3.2. Macro Anatomy
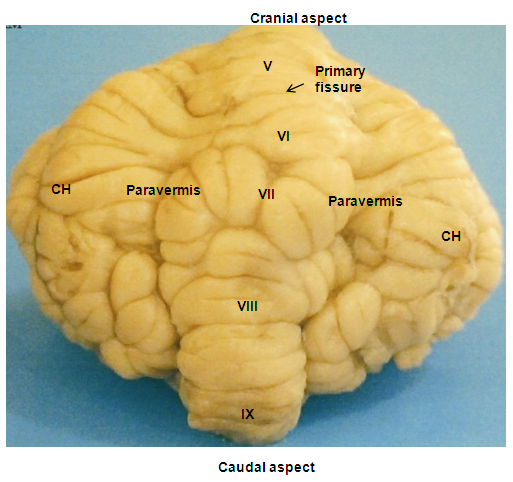
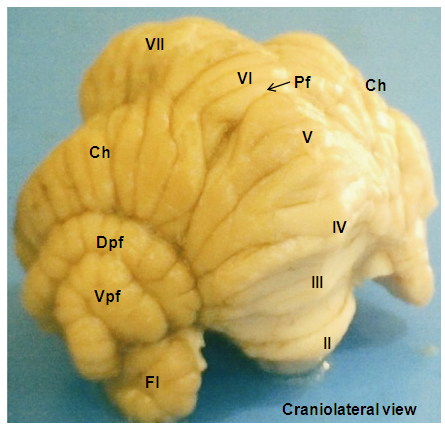
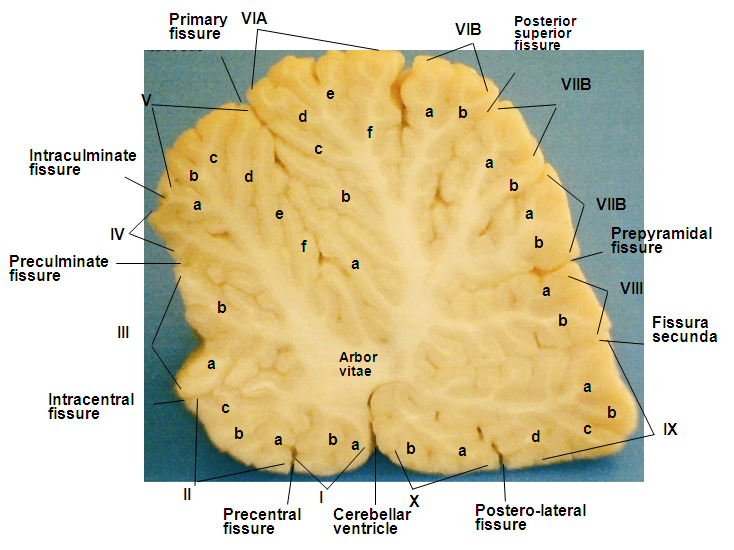
- The red Sokoto goat cerebellum was located on the dorsal aspect of medulla oblongata, bilaterally connected to it by cerebellar peduncles. The structure was composed of well-developed centrally located vermis, the bilateral cerebellar hemispheres (Hemispherium cerebelli) and well-defined paravermis regions, given rise to a globulous mass. The cerebellar cortical surface composed of numerous convolutions (Folia cerebelli), with intervening sulci (Sulci cerebelli) and fissures (Fissurae cerebelli) of varying depths, extended transversely across the vermian region to the bilateral cerebellar hemispheres (Plates 1, 2, and 3). The mid-sagittal view of the vermis revealed the three lobes; the rostral (Lobus rostralis), caudal (Lobus caudalis) and the flocculonodular lobe (Lobus flocculonodularis). The rostral and caudal lobes were separated by primary fissure (Fissura prima), which formed the bulk of the cerebellum (Corpus cerebelli). The Corpus cerebelli was composed of lobule I- to IX, and the lobule X (Nodulus) was part of the flocculonodular lobe, being connected to the flocculus. The flocculus was relatively small and elongated with slender stalk, the floccular peduncles (Pedunculus flocculi) connecting it to the nodulus. The arbor vitae was relatively wide (Plates 4 and 5).
3.3. The Vermal Lobules
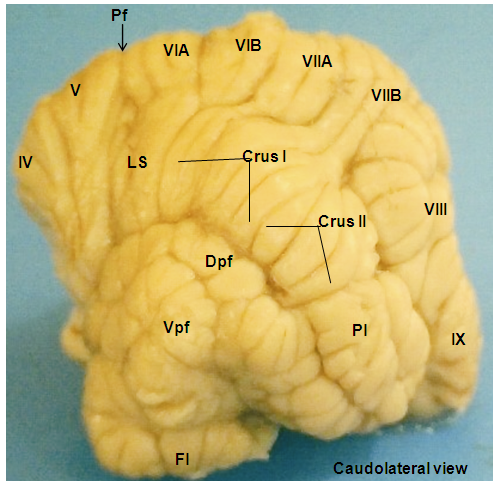
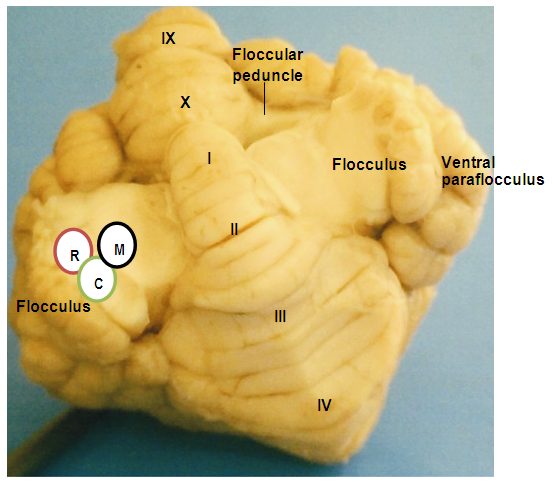
- In the rostral lobe, the lobule I (Lingula cerebelli) was relatively small, short and had two sublobules (Ia and Ib) slanted in the cranioventral direction. The shallow precentral fissure separated lobule I from lobule II. The lobule II (Lobulus centralis) was slightly larger than lobule I, it slanted cranioventrally with three sublobules (IIa and IIb and IIIc) separated by intracentral sulci. Separating the lobule II from lobule III was an intracentral fissure. The lobule III (Culmen: Pars rostralis) was broader than the previous lobules, and was sublobulated into IIIa, and IIIb by short sulci, with IIIb being slanted dorsally and IIIa ventrally. The three lobules (I, II, and III) were not visible on the dorsal view. The preculminate fissure separated lobule III from lobule IV, and seemed to be the deepest fissure in the rostral lobe (Plates 1 and 2). The lobule IV (Culmen: Pars caudalis) was small and slightly viewed dorsally, but without sublobule and the short intraculminate fissure separated it from lobule V. The lobule V (Declive) was the second longest and broadest lobule after lobule VI of the cerebellum viewed on the dorsal surface. It had six sublobules (Va, Vb, Vc, Vd, Ve and Vf) separated by small fissures and small sulci. The Fissura prima was the deepest fissure of the cerebellum in this species, it delimit lobule V from lobule VI (Plate 5). In the caudal lobe, the most complex lobule in this study was lobule VI (Folium vermis). It showed two parts; the VIA and its sublobules (a, b, c, d, e and f) and VIB with two sublobule (a & b). The Posterior superior fissure was long, slanted in a caudal direction and curved dorsally to separate lobule VI from lobules VII. Lobule VI constituted the vermian part of the Lobules simplex. Lobule VII (Tuber vermis) was found to be broad and subfoliated into two parts (VIIA and VIIB) like lobule VI, each having two sublobules (a & b). This lobule was caudally directed for most of its length, with its end turning slightly dorsal. The short pre-pyramidal fissure separated lobule VII from lobule VIII. The lateral representation of lobule VII was the ansoparamedian lobule. Lobule VIII, the Pyramis [vermis] fanned caudally and subfoliated into VIIIa and VIIIb by shallow sulci, the intra-pyramidal sulcus 1. Fissura secunda separated lobule VIII from lobule IX. Lobule IX, the Uvula [vermis] was broad and sublobules into IXa, IXb, IXc and IXd by uvular sulci 3, 2 and 1, respectively. A curved fissure, Postero-lateral fissure separated lobule IX from lobule X (Plate 4). The flocculonodular lobe was situated caudal to the uvula and separated rostrally from the Corpus cerebelli by the Fissura uvulonodularis. This smallest lobe was formed by two lobules; the vermian nodulus and a lateral flocculus on each side. The lobule X (Nodulus) was small, slanted ventrocaudally and sublobules into small Xa and Xb (Plate 5).
3.4. The Cerebellar Hemispheres
- The Lobule simplex was the lateral extension of the vermian lobule VI and its sublobules. The lateral representation of lobule VII was the ansoparamedian lobule that consisted of crus I (Crus rostrale), crus II (Crus caudale) and cranially the paramedian lobule (Lobulus paramedianus). The Crus I consisted of 3 broad lobules that tapered toward the vermis and united into a constricted isthmus, which continued into the vermian segment. The Crus II was observed to be divided into 3 lobules like the former by two furrows; one extended about halfway from the lateral surface to the midline and the other extended from the side of the hemisphere for only a short distance into the dorsal surface. The paramedian lobule consisted of four short folia that curved laterally and ventrally parallel to the folia of crus II. Medially, these folia converged and continued with the caudal part of lobule VIII, the pyramis, through a short lateral extension from the base of the pyramis (Plate 1). The paraflocculus was divided into a dorsal paraflocculus (Paraflocculus dorsalis) and a ventral paraflocculus (Paraflocculus ventralis), with the dorsal paraflocculus continuing medially into the pyramis and the ventral paraflocculus continued into the uvula. Each part of the paraflocculus had many folia, but the dorsal, in addition, was broader (Plate 3).
4. Discussion
- The cerebellum gross morphology in this study was similar to the general rule that characterized the mammalian cerebellar zones, that is, a centrally located vermis, the paravermis and the bilateral cerebellar hemispheres (Fletcher, 2006; Sultan and Braitenberg, 1993) [6, 12]. It was noted that though, the zonal pattern seems is similar, variations exist in relative size, as it relates to its functions among and within the vertebrates [13]. In this study, the vermis was relatively broad and the cerebellar hemispheres broader with prominent lateral paraflocculus. This was similar to the rat cerebellum reported by [14]. In humans and higher primates, very large cerebellar hemispheres were correlated to the independent use of individual extremities [15]. It has also been showed that the presence of large hemispheres of the Lobules simplex and ansiform lobules of primates is due to their dexterous forelimbs [14]. In the same vein, we have reported an enlarged vermis and cerebellar hemispheres in some African rodents, such as African giant rat where we correlated their sizes to the animal’s use of their limbs in the wild for climbing swimming and in feeding habit [16]. Research has shown that the size of individual folium or lobule is related to function, that is, the more complex and extensive the size of a folium, the better the function. A pioneer works on the cerebella mammals and avian showed that the expansions of individual lobules were correlated with behavioral differences among species [10]. On midsagittal section of the cerebellum in this study, we observed striking differences in the lobule sizes and their sublobulations. For example, both lingula and central lobule had two sublobules, and were not visible on the dorsal view. According to [9], these lobules (ligula and central lobule) are relatively large in mammals that have proportionately large and long tails, such as seen in the rat, but moderately developed in the pig which has a rather ineffectual tail. Earlier, in cerebellum research, these lobules were showed to be largest in spider monkey [17]. Tail in these monkeys served as a fifth limb, where they use them for maneuvering through the tree branches. The relatively small size of these lobules in the red Sokoto goat may be related to the small, short tail, which cannot be used to drive away flies from its body. These findings further support the argument that these lobules are related to the tail activities.In this research, the largest lobule in the rostral lobe was declive (lobule V). The size of this lobule has been reported to correlate with the movements of the lower extremities of the forelimbs [18]. Review on the functional topography of human cerebellum shown that task involving finger movements are associated with the function of lobule V [19]. These findings further support the correlation between the relatively broad size of lobule V in this study to the animal use of limbs during grazing in the field.The most complex lobules in this study (VI and VII) have well developed lateral extensions, like the Lobule simplex. Similarly, lobule VI has been reported to be the most complex also in African giant rat and second to lobule IX in the grasscutter [20]. Base on structure and function in mammals, lobules VI and VII are regarded as the oculomotor [21], because they receive proprioceptive, vestibular and auditory inputs and are involved in the guidance and modification of eye movements called saccade. Robinson and Fuchs (2001) [22] proved the cerebellum’s role in saccades from the study of the saccade-related part of the caudal lobe of the vermis, or the oculomotor vermis. In another research, [23] noted that Crus I and crus II of the ansiform lobe of the cerebellum in rats receive signals from peri-oral structures such as lips, teeth and vibrissae. In our present study, these regions (lobules VI and VII) were well formed, and may be related to the ability of red Sokoto goats to use these organs in the ecosystem. The cerebella of these animals in this work had large paraflocculus located beneath the lateral portions of the cerebellar hemispheres. The primates with increasing upright position and man with independent use of individual extremities have well developed cerebellar hemispheres, similar to our findings [24]. The feeding habit of the red Sokoto goat to has been described to include, consumption of relatively dry leaves and standing hay than other breeds [25]. In addition, it shows great interest in climbing trees and uncompleted buildings. This behaviour may be correlated to the well-developed bilateral cerebellar hemispheres and paraflocculus, in agreement with [24].In the red Sokoto cerebellum, the flocculus was entirely separated except for the stalk of attachment to the nodulus, corresponding with the findings of [9] for other mammals. Functionally, the flocculo-nodular lobe of the cerebellum said to be involved in the control of eye movements, head movements, and balance [6]. This lobe is termed the “vestibular cerebellum” because its major input projections are from the primary vestibular afferents. In humans, it’s a pretty small structure, nevertheless, it is important. In fish and birds, the vestibular cerebellum is the largest part of the cerebellum [26].
5. Conclusions
- The well-developed bilateral cerebellar hemispheres with large paraflocculus in the cerebellum of red Sokoto goat may be related with high motor activities; such as climbing, standing erect on hindlimbs while the forelimb is suspended in the air to grasps leaves of short trees during feeding in their ecological zones.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML