-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2017; 6(3): 43-50
doi:10.5923/j.ijbcs.20170603.01

Flaxseed Oil as a Potential Neuro-protective Agent on the Cerebellum of Rotenone Mice Model of Parkinson’ Diseases
Philemon Dauda Shallie1, Damilola Joseph Talabi1, Olugbenga Olawole Olayinka1, Bamidele Richard Babatunde1, Helen Bassey Akpan1, Olutoye Jibrin Otulana1, Oluwole Ojo Alese2, Oluwadamilola Faith Shallie1
1Department Anatomy, Olabisi Onabanjo University, Nigeria
2Department Physiology, University of Kwa Zulu Natal, South Africa
Correspondence to: Philemon Dauda Shallie, Department Anatomy, Olabisi Onabanjo University, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Pathological changes in the cerebellum following dopaminergic degeneration were reported in patients with Parkinson’s disease and animal models. A growing body of evidence suggests that nutrition may play an important role in PD. However, inclusion or exclusion of other food groups may trigger or exacerbate neurodegeneration. This study investigated the potential neuro-therapeutic effect of Flaxseed oil on the cerebellum of Rotenone mice model of Parkinson’ diseases’. Fifty-six adult male and female mice (Mus musculus) weighing between 23.9-26.3 grams were used for this study. The mice were randomly placed into four groups of seven mice each: A (Control; mice pellets), B (Rotenone 3mg/kg, IP), C (Rotenone + Flaxseed oil 0.3ml orally), and D (0.3ml Flaxseed + Rotenone). At the end of the experimental period, the animals were subjected to open field test and subsequently sacrificed by 10mg/kg of pentobarbital. The brain were excised, weighed and appropriate sections taken and processed histology and stained H&E and Nissl and immune-cytochemically for neurofilaments. Results: The results showed significant (P<0.005) increase in anxiety in the rotenone group which were lowered by flaxseed oil treatments. Rotenone induced cerebellar cortical neural derangements which were ameliorated by flaxseed oil treatment. Conclusion: In conclusion, the increase anxiety by rotenone induced Parkinson’s disease in this study were reduced or alleviated with flaxseed oil treatment; hence it could be considered as a neuro-protective candidate in Parkinson's disease induced neuronal insult.
Keywords: Flaxseed oil, Rotenone, Cerebellum, Parkinson’s disease
Cite this paper: Philemon Dauda Shallie, Damilola Joseph Talabi, Olugbenga Olawole Olayinka, Bamidele Richard Babatunde, Helen Bassey Akpan, Olutoye Jibrin Otulana, Oluwole Ojo Alese, Oluwadamilola Faith Shallie, Flaxseed Oil as a Potential Neuro-protective Agent on the Cerebellum of Rotenone Mice Model of Parkinson’ Diseases, International Journal of Brain and Cognitive Sciences, Vol. 6 No. 3, 2017, pp. 43-50. doi: 10.5923/j.ijbcs.20170603.01.
Article Outline
1. Introduction
- Pathological changes in the cerebellum following dopaminergic degeneration were reported in patients with Parkinson’s disease and animal models. Rolland showed that degeneration of nigrostriatal dopaminergic neurons causes dysfunction of both the basal ganglia–thalamic and cerebello-thalamic pathways in 6-hydroxydopamine-lesioned rats and MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) monkeys [1]. Neuronal degeneration in the cerebellum was shown in an MPTP mouse model [2], characterized by the loss of Nissl stained Purkinje cells and aggravated by the number of repeated MPTP injections. An MPTP insult also induced the loss of calcium-binding positive Purkinje cells in monkeys [3]. While cerebellar dysfunction might contribute to some motor and non-motor signs in Parkinson’s disease, a possible approach for treating parkinsonian symptoms is to attempt to normalize cerebellar function. Surgical treatment, such as deep brain stimulation of the subthalamic nucleus [4-8] or globus pallidus [9] improves the motor signs and normalizes cerebellar activation. Levodopa administration can also normalize the activity and connectivity in the cerebello-thalamo-cortical circuit [10, 11]. However, whether it is reduced compensation or alleviation of pathological impairment as a consequence of effective treatment remains unclear. Suppressing cerebellar activity should theoretically answer the question: improvement would mean that the cerebellum is contributing to the manifestations; worsening would mean that the cerebellar activity is compensatory. We therefore investigated the protective or ameliorative effect/s of flaxseed oil on the morphology of the cerebellum of rotenone mice model of Parkinson’s disease.
2. Materials and Methods
2.1. Experimental Animals
- Fifty-six adult male and female mice (Mus musculus) weighing between 23.9-26.3g were used for this study. The animals were housed in clean plastic cages, well ventilated environment with temperature ranging between 24-28°C in 12 hours light and 12 hours dark cycle. The animals were given standard mice pellets and water ad libitum, and were allowed to acclimatize for two weeks before commencing the experimental protocols. The institutional committee on Animal Care and Use in Research, Education and Testing (ACURET) approval was obtained and the animal experiments were conducted according to the NIH Guide on Laboratory Animals for Biomedical Research (NIH, 1978) and ethical guidelines for investigation of experimental pain in conscious animals [12].
2.2. Experimental Design
- Following the two weeks of acclimatization, the animals were randomly divided into four (4) groups of fourteen (14) animals each made up of equal number of male and female mice as follows:Ÿ Group A: (Control Group) Mice were given dry food pellet and clean water ad libitum.Ÿ Group B: (Negative Control Group) Mice were given 3 mg/kg/day of Rotenone per body weight subcutaneously for 5 consecutive daysŸ Group C: (Post-treated) Mice were given 3 mg/kg/day of Rotenone per body weight subcutaneously for 5 consecutive days followed by a fourteen (14) days oral treatment with (0.3mil/mouse) flax seed oil.Ÿ Group D: (Pre-treated) Mice were given (0.3mil/mouse) flax seed oil for fourteen (14) days consecutively followed by five (5) days administration of 3 mg/kg/day of Rotenone subcutaneously.
2.3. Induction of Parkinson’s Disease
- Subcutaneous Implantation of Osmotic Pumps were filled with rotenone (abcam) dissolved in DMSO to make a final concentration of 3.0mg/kg/day for 5 consecutive days. Osmotic minipumps were implanted under the skin on the back of each animal [13]. Treated mice received 3.0 mg of rotenone/kg/day. The animals were sacrificed on the 6th day after surgery.
2.4. Open Field Exploration Test
- After 6 weeks of experimental examination, another test was carried out, the open field test. This was done to test for the effect of the rotenone induced on the mice memory.
2.4.1. The Open Field Test
- The open field can be of different sizes; small (38 x 38 cm), or large (72 x 72 cm). The small open field can also serve as a whole board and as a test chamber for the novel object recognition task. The large open field is used for measuring anxiety and exploration as well as locomotion as it has a large center arena.
2.4.2. Apparatus
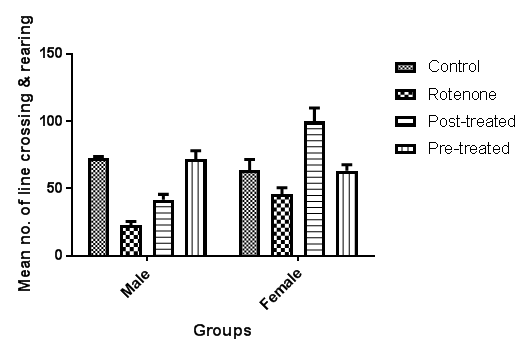
- The open field apparatus was constructed of white plywood and measured 72 x 72 cm with 36 cm walls. One of the walls was clear Plexiglas, so mice could be visible in the 2 apparatus. Blue lines were drawn on the floor with a marker and were visible through the clear Plexiglas floor. The lines divided the floor into sixteen 18 x 18 cm squares. A central square (18 cm x 18 cm) was drawn in the middle of the open field [14]. The central square is used because some mouse strains have high locomotor activity and cross the lines of the test chamber many times during a test session. Also, the central square has sufficient space surrounding it to give meaning to the central location as being distinct from the outer locations [15]. The maze was located in a 1.8 x 4.6 m test room and lit by a 60-watt red lamp for background lighting. The open field maze was cleaned between each mouse using 70% ethyl alcohol. Behavior was scored with Hindsight for MS-dos (ver 1.5), and each trial was recorded for latter analysis, using a video camcorder (Hitachi, VM-7500LA) positioned above the apparatus. Measures of line crosses were obtained with an automated camera-based computer tracking system (Limelight, Actimetrics) on an IBM PC computer with the camera fixed to the ceiling, 2.1 m above the apparatus.
2.4.3. Procedure
- Mice were carried to the test room in their home cages and were handled by the base of their tails at all times. Mice were placed into the center, or one of the four corners of the open field and allowed to explore the apparatus for 5 minutes. After the 5 minute test, mice were returned in their home cages and the open field was cleaned with 70% ethyl alcohol and permitted to dry between tests. To assess the process of habituation to the novelty of the arena, mice were exposed to the apparatus for 5 minutes on 2 consecutive days.
2.4.4. Behaviours Scored
- 1. Line Crossing: Frequency with which the mice crossed one of the grid lines with all four paws. 2. Center Square Entries: Frequency with which the mice crossed one of the red lines with all four paws into the central square. 3. Center Square Duration: Duration of time the mice spent in the central square. 4. Rearing: Frequency with which the mice stood on their hind legs in the maze. 5. Stretch Attend Postures: Frequency with which the animal demonstrated forward elongation of the head and shoulders followed by retraction to the original position. 6. Grooming: Duration of time the animal spent licking or scratching itself while stationary. 7. Freezing: Duration with which the mouse was completely stationary. 8. Urination: number of puddles or streaks of urine. 9. Defecation: number of fecal boli produced. Each animal was then given a score for total locomotor activity that was calculated as the sum of line crosses and number of rears.
2.5. Tissue Sample Preparation
- At the end of the experimental period the mice were euthanized by administering 10g/kg body weight of Pentobarbital. The mice brains were carefully dissected out, weighed and fixed in 10% formol-saline for routine histological and immunocytochemical procedures.
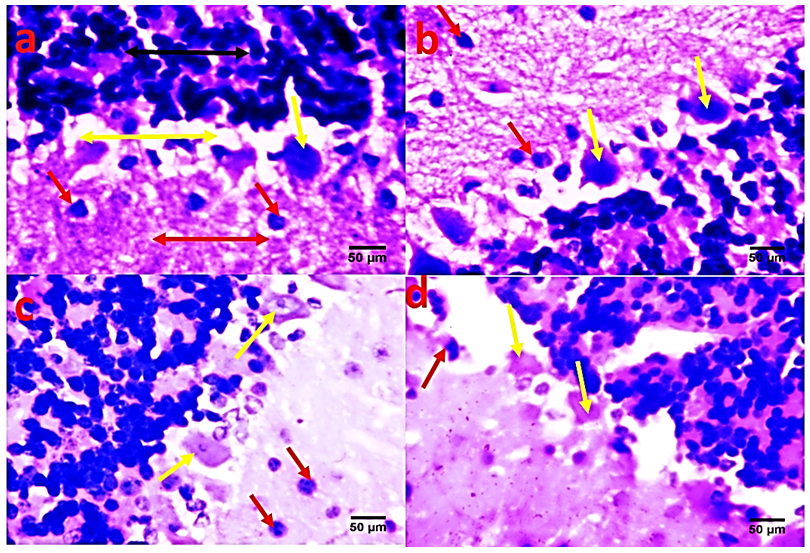
2.5.1. Haematoxylin and Eosin Routine Staining
- Tissue sections were rinsed in distilled water for 5 minutes, then stained in haematoxylin for 15 minutes, rinsed in running tap water and differentiated in 0.3% acetic acid and rinsed in tap water before staining with eosin for 2 minutes. Sections were then dehydrated in 70% for 1 minute, 95% alcohol for 1 minute, 100% alcohol for 1 minute (2 changes) respectively and then taken to the oven overnight. Sections were subsequently cleared in xylene and then placed DPX mountant and cover slipped for light microscopy [16].
2.5.2. Bielschowsky's Silver Staining Protocol
- Deparaffinized sections to distilled water and washed three times, the slides were pre-warmed (40°C) and stained in 10% silver nitrate solution for 15 minutes, the slides were then placed in distilled water and washed for 3 times; added to the silver nitrate solution, was concentrated ammonium hydroxide drop by drop until the precipitate formed was JUST clear. The slides were placed back in this ammonium silver solution and stained in 40°C oven for 30 minutes or until sections become dark brown, slides were placed directly in developer working solution for about 1 minute, after this slides were dipped for 1 minute in 1% ammonium hydroxide solution to stop the silver reaction. Slides were then washed in distilled water in 3 changes. Slides were then placed in 5% sodium thiosulfate solution for 5 minutes, followed by yet another 3 changes of washing in distilled water. The sections were dehydrated and cleared through 95% ethyl alcohol, absolute alcohol and xylene and mounted with resinous medium [17].
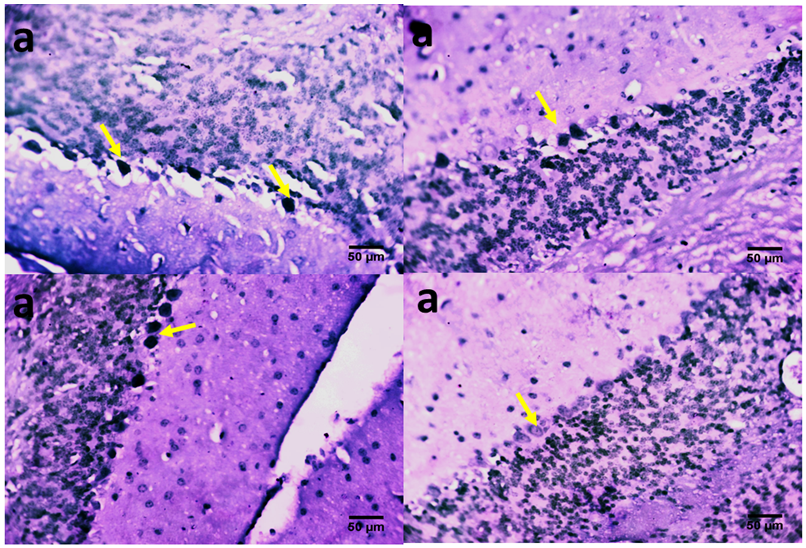
2.5.3. Methods: Cresyl Fast Violet for Nissl Substance
- Tissue sections were de-waxed in xylene (2 or 3 changes of 3min each), dehydrated in alcohol (100% x2), 3min each, followed by staining in 0.1% Cresyl Violet for 15min. The slides were quickly rinsed in tap water to remove excess stain, then washed in 70% ethanol, followed by dehydration through 2x3min changes of absolute ethanol and finally cleared in xylene x2 and mounted in DPX [18].
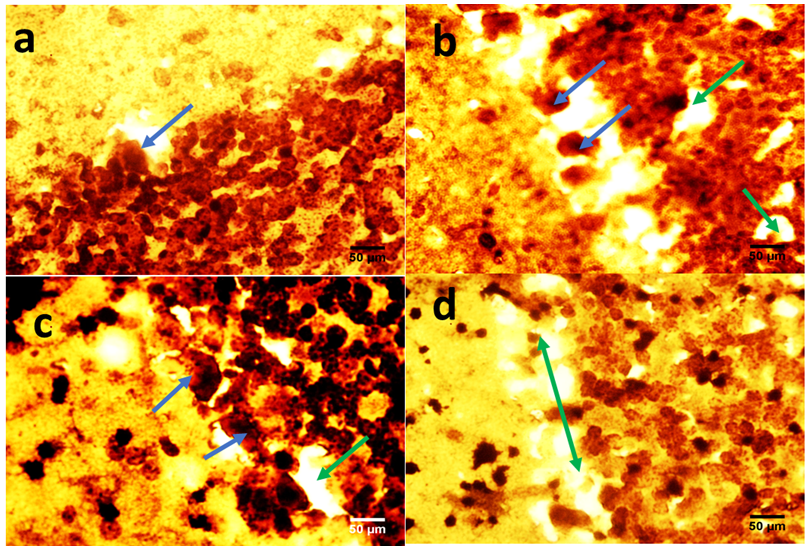
2.6. Immunohistochemical Protocol
- The paraffin embedded tissue was cut at 5 microns thick and allowed to heat on hot plate for 1 hour, then sections were taken to water, that is, through xylene, alcohols and finally water respectively. Antigen retrieval method was performed using citric acid solution pH 6.0 in a pressure cooker for 15 minutes. Sections were equilibrated by gently displacing hot citric acid with running tap water for 3 minutes. Blocking of peroxidises in tissue sections was done using peroxidise block for 15 minutes and then washed for 2 minutes with phosphate buffered saline (PBS) with tween 20. Blocking of protein was then performed with Novocastra® protein block for 15 minutes. Tissue section was then washed for 2 minutes with PBS, then incubated with primary antibody e.g., Neurofilaments 1 in 100 dilution for 45 minutes, washed in PBS for 3 minutes and later added Secondary antibody for 15 minutes. Tissue section was then washed twice with PBS. Polymer was thereafter added and allowed for 15 minutes, washed twice with PBS and then added the diaminobenzidine (DAB) chromogen diluted 1 in 100 with the DAB substrate for 15 minutes, and then washed with water and counterstained for 2 minutes in Haematoxylin. Again the tissue section was washed, dehydrated, cleared and mounted in DPX mountant [19].
2.7. Photomicrography
- Photomicrographs were taken using Omax led digital Microscope.
2.8. Statistical Analysis
- Data were analysed using analysis of variance (ANOVA) by comparing values for different treatment groups with the values for individual controls. Results were expressed as mean ± SEM. The significant differences among values were analysed using Graph Pad version 7 at P-value = 0.05.
3. Results
4. Discussion
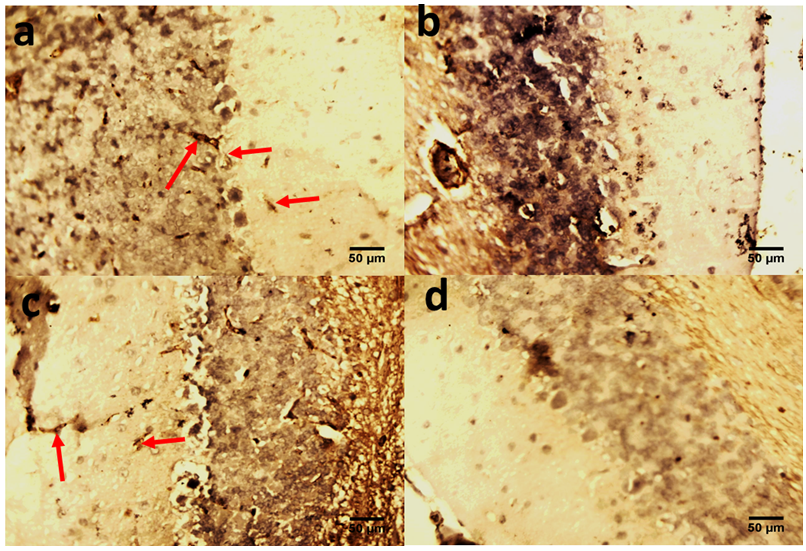
- The role of the cerebellum in Parkinson's disease includes two aspects, pathological and compensatory effects. Pathological changes in the cerebellum might be induced by dopaminergic degeneration, abnormal drives from the subthalamic nucleus and dopaminergic treatment, and may account for several clinical symptoms in Parkinson's disease. Because dopaminergic degeneration develops gradually [20], presumably, pathological impairments should be more severe as disease progresses. The compensatory effect may help to maintain relatively normal motor and non-motor function. Our results showed that rotenone induced mice model of Parkinson’s disease presented with significantly reduced exploratory behaviour and locomotion which indicate increased anxiety [Fig. 1]. Decreased anxiety leads to increased exploratory behaviour. Increased anxiety will result in less locomotor motion and preference for the edges of the field [21]. Anxiety in PD is attributed to a combination of medical, neurochemical and psychosocial phenomena. In a subset of patients, anxiety disorders are a ‘reactive’ response secondary to the diagnosis of PD. However, when compared with non-PD patients with chronic illnesses and similar disability, anxiety in patients with PD is significantly more severe [22]. Epidemiologic observations indicate that patients with PD are at greater risk of developing anxiety disorders before the diagnosis of PD [23, 24]. These findings suggest that anxiety may be an early nonmotor phenotype of PD and that disability, although it may contribute to anxiety, is not the sole etiologic determinant. Episodic anxiety has been associated with motor fluctuations [25-27]. We also investigated the impact of the treatment on the microstructure of the cerebellum; our findings showed that rotenone induced neuronal derangements in the rotenone only and the post-treated groups [Plates 1, C & C; Plate 3, B & C], Immuno-histochemical examinations, also revealed that neurofiliments were completely depleted in the rotenone only group [Plate 4, B], while. Pathological changes in the cerebellum following dopaminergic degeneration were reported in patients with Parkinson's disease and animal models. Rolland showed that degeneration of nigrostriatal dopaminergic neurons causes dysfunction of both the basal ganglia–thalamic and cerebello-thalamic pathways in 6-hydroxydopamine-lesioned rats and MPTP (1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine) monkeys [1]. Neuronal degeneration in the cerebellum was shown in an MPTP mouse model [2], characterized by the loss of Nissl-stained Purkinje cells and aggravated by the number of repeated MPTP injections. An MPTP insult also induced the loss of calcium-binding positive Purkinje cells in monkeys [3]. A recent study found that persistent hyper-activation of Purkinje cells correlated with the level of dopaminergic neuronal loss in the substantia nigra in chronic parkinsonian monkeys [4]. Our present study reported that both pre and post-treatment with flax omega 3 in the form of flaxseed oil significantly reduced anxiety in the mice as shown by the significant increase in rearing and locomotion [Fig.1] and apperant features of neuroprotection [See Plates 1, D; Plate 2, C & D; Plate 3, D and Plate 4, C & D]. These results are suggest that omega 3 consumed in the form of flaxseed oil might be an effective supplement for the prevention of neurodegenerative diseases which are associated with oxidative stress. DHA has been reported to scavenge the intracellular radical productions induced by hydrogen peroxide (H2O2), superoxide anion (O2•−), and hydroxyl radical (•OH) [28]. Many previous studies reported that DHA treatment could significantly reduce ROS production, which is a possible mechanism underlying DHA’s protective effects [28].In conclusion, the increase anxiety and neuronal derangements associated with rotenone induced Parkinson’s disease in this study were reduced or alleviated as a consequence of the treatment with flaxseed oil. These outcome demonstrated that the cerebellum is a potential target to relieve some Parkinson's disease symptoms.
ACKNOWLEDGEMENTS
- We want to acknowledgment Mr Stanley Izobo, the chief technologist for his technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML