-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2017; 6(1): 9-16
doi:10.5923/j.ijbcs.20170601.02

Histopathological Effect of Nauclea latifolia Ethanolic Leaf Extract and Artemether/Lumefantrine on the Hippocampus of P. berghei-Infected Mice
Innocent A. Edagha, Aniekan I. Peter, Aquaisua N. Aquaisua
Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
Correspondence to: Innocent A. Edagha, Department of Anatomy, Faculty of Basic Medical Sciences, University of Uyo, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objective: Severe malaria and cerebral malaria reportedly causes cognitive deficits in young children, post-treatments. We investigated the antiplasmodial, histomorphological and immunohistochemical effects of ethanolic leaf extract of Nauclea latifolia (NL) on the hippocampus of Swiss albino mice infected with Plasmodium berghei. Methods: Twenty male mice were divided into four groups of five mice each as follows: Group 1 (control) was given 10 ml/kg of normal saline (0.9% Nacl); Group 2 received extract of NL 500 mg/kg body weight of mice. Group 3 received 1000 mg/kg body weight of mice. Group 4 received 5 mg/kg body weight of mice. All mice were passaged with the 0.2ml of infected erythrocytes containing approximately 1x106 of P. berghei via intraperitoneal injection before treatment. Baseline parasitemia was determined by direct enumeration on day 5 post infection, then treatment commenced for five days, thereafter mice were humanely sacrificed via ketamine/xylaxine cocktail, and brain dissected for light microscopy, and immunohistochemical detection of glial fibrillary acidic protein (GFAP) expression in the hippocampus. Result: P. berghei infection was significantly (p<0.05) decreased in NL ethanolic extract groups at dose dependent levels, but decreased most in the Artemether/Lumefantrine groups. Histomorphology revealed severely distorted hippocampus in group 1 (P. berghei-infected only); petechial haemorrhage in the low dose extract, but ameliorated distortions in NL 1000 mg/kg and 5mg Artemether/Lumefantrine groups. GFAP was intensely expressed in group 1, moderate in 2, but down-regulated in group 3 and 4 indicating a decline in induced oxidative stress to neuronal cells and neuropil by the extract and Artemether/Lumefantrine. Conclusion: The ethanolic extract of NL via its phytochemical bioavailability decreases P. berghei in dose dependent manner, and offers moderate neuro-protection to hippocampus of infected mice, down-regulating inflammatory protein in curative malaria model; thus may support their wide use in ethnopharmacology, we are investigating the activity of their fractions and pure compounds in other antimalarial models.
Keywords: Cerebral malaria, Nauclea latifolia, Artemether/Lumefantrine, Hippocampus, Astrogliosis
Cite this paper: Innocent A. Edagha, Aniekan I. Peter, Aquaisua N. Aquaisua, Histopathological Effect of Nauclea latifolia Ethanolic Leaf Extract and Artemether/Lumefantrine on the Hippocampus of P. berghei-Infected Mice, International Journal of Brain and Cognitive Sciences, Vol. 6 No. 1, 2017, pp. 9-16. doi: 10.5923/j.ijbcs.20170601.02.
Article Outline
1. Introduction
- As far back as 2011, an estimated 50% of the world’s population were reportedly at risk of malaria infection, with populations living in sub-Saharan Africa having the highest risk of acquiring malaria: approximately 80% of cases and 90% of deaths are estimated to occur in the WHO African Region, with children under 5 years of age and pregnant women most severely affected [1], efforts have since been made in malaria vaccine discovery, alongside alternative therapy in the event of widespread artemisinin-resistant malaria. Malaria has had the largest impact of any infectious disease on shaping the human genome, exerting enormous selective pressure on genes especially in severe malaria infections [2].Plasmodium berghei is a model which is commonly used to study human malaria, the average protein identity between P. falciparum and P. berghei is 62.9% and their average nucleotide identity is 70.3% [3]. Although, strain-specific, it has been reported that between 90 and 100% of P. berghei infected mice succumb to cerebral malaria (CM) between days 6 and 8 post infection with moderate to high parasite, and mice have been considered to have CM if they displayed neurological symptoms such as paralysis, deviation of the head, ataxia, convulsions and coma [4]. P. berghei causes malaria in mice [5] and have served as surrogate models of human malaria in the fields of pathology, immunology, genetics, molecular biology and biochemistry. Nauclea laltifolia Smith (family: Rubiaceae) occur wildly in Savanna forests of continental Africa and has been used by the natives of East and West Africa in traditional medicine for treatment of various ailments [6]. It is a common plant in the tropical forests of South Southern and South Eastern areas of Nigeria. Rubiaceae is a large family of 630 genera and about 13000 species found worldwide, especially in tropical and warm regions [7]. The leaves are used for the treatment of malaria in East Africa [8] and in Nigeria [9], and because of its reported anti-malarial activity, the plant has been known as ‘African cinchona’ or ‘African quinine’ [10]; Cold water extracts of the leaves of Nauclea latifolia as well as some of its fractions were active against P. berghei in vivo [11]. Nauclea latifolia is active against P. falciparum [12]. A root method and composition for treating malaria under the US patent application publication [13]; antiplasmodial activity from root bark ethanolic extracts on in vitro [14]. NIPRD-AM1 is an herbal medicine developed at the National Institute for Pharmaceutical Research and Development (NIPRD) Abuja, Nigeria, from aqueous root extracts of Nauclea latifolia for the treatment of uncomplicated malaria. The physicochemical and other quality variables of NIPRD-AM1 have been reported [15]. The best understood functions of the hippocampus are the retention of information in short- term memory and its transfer into long- term declarative memory [16]. Cognitive dysfunction is sustained after rescue therapy in experimental cerebral malaria, and is reduced by additive antioxidant therapy [17]. In this study we investigate the antiplasmodial, histomorphological and immunohistochemical effects of ethanolic leaf extract of Nauclea latifolia (NL) and Artemether/Lumefantrine on the hippocampus of Swiss albino mice infected with Plasmodium berghei.
2. Materials and Methods
- Experimental AnimalsTwenty male Swiss albino mice weighing 20-24g were obtained from the animal house of the Faculty of Basic Medical Sciences, University of Uyo, Nigeria. The animals were acclimatized for two weeks prior to the start of the experiment in the institution’s animal holding room in well ventilated cages and maintained under controlled environmental conditions of temperature 25±5°C and 12 hour light/dark cycle. All the animals were allowed access to feed (rat mash; Vital Feeds from Grand Cereals Limited, Jos, Plateau State), and water ad libitum. All procedures involving animals in this study conformed to the guide for the care and use of laboratory animals [18] and granted approval by the Department of Anatomy ethical committee, University of Uyo.Collection and Authentication of Plant SampleFresh leaves of Nauclea latifolia obtained from the medicinal farm of Pharmacology and Toxicology Department, University of Uyo were identified and authenticated by the Curator at the Herbarium of Department of Pharmacology and Toxicology, University of Uyo with specimen and voucher number UUH/67 (g) deposited.Plant ExtractionFresh leaves of Nauclea latifolia macerated in 95% ethanol (Sigma Aldrich St Louis USA) in a flat bottom flask and kept for 72 hours at room temperature. The macerated leaves were filtered and the filtrate concentrated in water-bath at 45 degree Celsius to dryness. Yield of extract was weighed and stored in the refrigerator until required for use.Parasite Inoculation P. berghei was obtained commercially from National Institute of Medical Research (NIMER), Yaba, Lagos, Nigeria. Twenty mice were inoculated intraperitoneally with 0.2 ml of infected blood containing about 1×106 Plasmodium berghei parasitized erythrocytes. The inoculums consisted of 5×106 Plasmodium berghei erythrocytes per ml. This was prepared by determining both the percentage parasitaemia and the erythrocytes count of the donor mouse and diluting the blood with isotonic saline in proportions indicated by both determinations [19].Drug administrationArtemether/Lumefantrine (Coartem®) Novartis was purchased from a reputable pharmacy within the Uyo metropolis. A tablet was dissolved in distilled water and dosage administered according to method by [20], and administered via oro-gavage intubation based on body weights. Stained thick blood smears obtained from the tail vein of the mice was made and viewed under oil immersion at x100 magnification; thereafter images on plates were obtained using a digital microscopic camera.Evaluation of Curative Activity of Extract and Drug (Rane’s test)This was used to evaluate the schizontocidal activity of the NL ethanolic extract and Artemether/Lumefantrine (Coartem®) in established infection, and this was done as described [21]. P.berghei berghei was injected intraperitoneally into twenty (20) mice on the first day (DO). Seventy-two hours later (D3), the mice was divided randomly into four groups of five mice each. Group 1 served as the control and received 10 ml/kg of normal saline (0.9% Nacl), group 2 received 500 mg/kg of ethanolic leaf extract, group 3 received 1000 mg/kg of ethanolic leaf extract, and group 4 received 5 mg/kg of Coartem®. The extract and drug were administered once daily for 5 days. Giemsa stained thick smears were prepared from tail blood samples collected on each day of treatment to monitor parasitaemia level. Tissue ProcessingMice were fasted overnight before being humanely sacrificed with a cocktail of ketamin/xylaxine 0.2ml. The brain were perfused with buffered saline and buffered formalin via a drip set up controlled by a valve, and then allowed to fix for at least 72 hours before they were dissected out and dried on a filter paper. Brain specimens were then processed to reveal the structures required for investigation. Haematoxylin and Eosin Routine Staining Tissue sections were rinsed in distilled water for 5 minutes, then stained in haematoxylin for 15 minutes, rinsed in running tap water and differentiated in 0.3% acetic acid and rinsed in tap water before staining with eosin for 2 minutes. Sections were then dehydrated in 70% for 1 minute, 95% alcohol for 1 minute, 100% alcohol for 1 minute (2 changes) respectively and then taken to the oven overnight. Sections were subsequently cleared in xylene and then placed DPX mountant and cover slipped for light microscopy [22].Immunohistochemical Protocol The paraffin embedded tissue was cut at 5 microns thick and allowed to heat on hot plate for 1 hour, then sections were taken to water, that is, through xylene, alcohols and finally water respectively. Antigen retrieval method was performed using citric acid solution pH 6.0 in a pressure cooker for 15 minutes. Sections were equilibrated by gently displacing hot citric acid with running tap water for 3 minutes. Blocking of peroxidises in tissue sections was done using peroxidise block for 15 minutes and then washed for 2 minutes with phosphate buffered saline (PBS) with tween 20. Blocking of protein was then performed with Novocastra® protein block for 15 minutes. Tissue section was then washed for 2 minutes with PBS, then incubated with primary antibody e.g., Glial fibrillary acidic protein (GFAP) 1 in 100 dilution for 45 minutes, washed in PBS for 3 minutes and later added Secondary antibody for 15 minutes. Tissue section was then washed twice with PBS. Polymer was thereafter added and allowed for 15 minutes, washed twice with PBS and then added the diaminobenzidine (DAB) chromogen diluted 1 in 100 with the DAB substrate for 15 minutes, and then washed with water and counterstained for 2 minutes in Haematoxylin. Again the tissue section was washed, dehydrated, cleared and mounted in DPX mountant [23].
3. Statistical Analysis
- Data obtained from the study was expressed as mean ± standard error of mean (SEM) and analyzed using one-way analysis of variance (ANOVA) to determine the difference between the experimental groups and the control group, and the post-hoc test (Student-Newman Keuls) for comparison, and values (p<0.05) was considered statistically significant.
4. Result
- The results obtained are presented in Tables 1 and 2; and Figures 1 A to 1D, 2A to 2D and 3A to 3D. Table 1 shows effect of ethanolic leaf extract of Nauclea latifolia and Artemether/Lumefantrine (Coartem®) on organosomatic index of P.berghei-infected mice which was not statistically significant (p>0.05), though there was apparent slight increase in percentage body weight in treated groups compared to control. Table 2 indicates a decrease in parasitemia and percentile chemosuppression was statistically significant (p<0.05) in the treated groups compared to control. Figure 1 (A to D) reveals Giemsa stained thick blood smear with evidence of parasite clearance in treated groups (1B-1D) compared to control (1A). Figure 2 (A to D) presents paraffin section H&E stained hippocampal CA1 region: 2A had few pyramidal neuronal shrinkage, some pyknosis, prominent vacuolations across the three layers of the hippocampus, and vascular congestion; 2B show petechial haemorrhage with neuronal hypertrophy with signs of oedema and some neuronal polymorphism; 2C has slight palely stained neuropil with mild hypotrophy; 2D has prominent neuronal shrinkage across the three layers and mild signs of lesion in the neuropil. Figure 3 (A to D) presents immunohistochemical labelling of glial fibrillary acidic protein (GFAP); 2A and 2B showed severely reactive astrogliosis; 2C was mild to moderate immunoreactivity, whereas 2D had very mild or the least detectable GFAP expression.
|
|
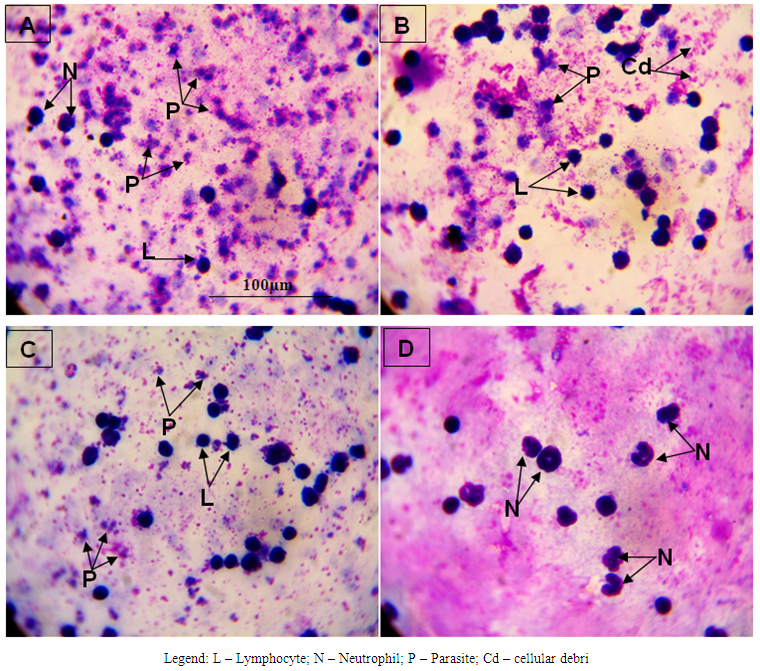 | Figure 1. Blood Morphological effect of Nauclea latifolia ethanolic leaf extract and Artemether/Lumefantrine (Coartem®) in Plasmodium berghei-infected mice |
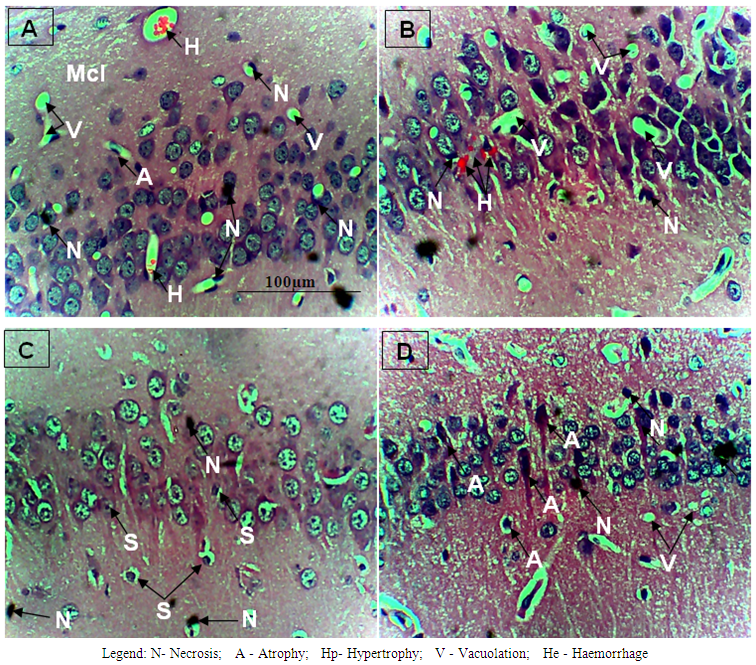 | Figure 2. Histomorphological effect of Nauclea latifolia ethanolic leaf extract and Artemether/Lumefantrine (Coartem®) in Hippocampus of Plasmodium berghei-infected mice |
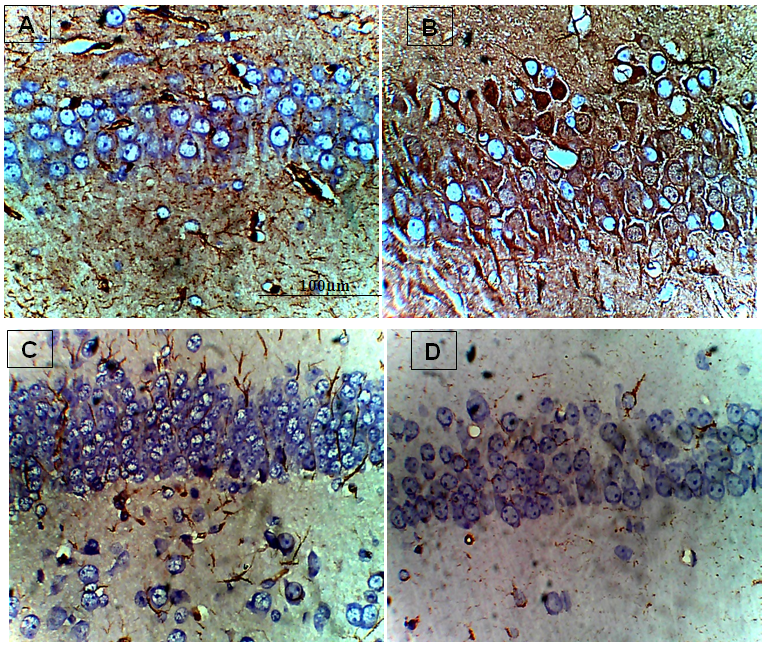 | Figure 3. Immunohistological effect of Nauclea latifolia ethanolic leaf extract and Artemether/Lumefantrine (Coartem®) in Hippocampus of Plasmodium berghei-infected mice |
5. Discussion
- In this study, Table 1 showed a dose dependent decrease in the body weight, although the organosomatic index was not significantly different in the treated compared to the control. A similar report of a dose-dependent reduction in the body weights of rats administered root extract of N. latifolia compared with the control group [24]. Reductions in weight may be as a result of the bioactive effect of alkaloids on weight loss [25]. Decrease in body weight of malarial mice has been reported [26], they noted that weight reduction in mice was clearly evident from the third day of infection, and presumably due in part to the decrease in food intake.Table 2 show that parasitemia is dose-dependently cleared in P. berghei-infected mice as similarly reported elsewhere [27], the thick blood smear show the level of parasite infectivity as presented in Figure 1 (A to D) with Figure 1A showing high parasitemia, but 1D had total parasite clearance (at least undetectable level of parasitemia). The phytochemical screening of the ethanolic leaves of N. latifolia indicates high concentrations of alkaloids, flavonoids, saponins, tannins and terpenes, but the absence of anthraquinones, deoxy-sugar and cardiac glycosides. [29] has reported high presence of alkaloids, flavonoids, tannins phenols, carbohydrate and saponins, and plants that contain bioactive compounds like alkaloids, flavonoids and triterpenoids may in part contribute their plasmocidal activity and therefore explain their mechanism of action [30]. Flavonoids are reported to chelate with nucleic acid base pairing of the Plasmodium parasite [31]. Phytochemical compounds like these elevate oxidation and inhibit the parasite’s protein synthesis [32], and by this could nullify the oxidative damage induced by the malarial parasite and this suggests that the antiplasmodial activity of N. latifolia ethanolic leaf extract may be based on the antioxidant and plasmocidal effects of these phytochemicals [33]. These corroborate the reported anti-plasmodial activity of the plant and thus may support their traditional use.In Figure 2 (A to D) is presented paraffin section H&E stained hippocampal CA1 region: 2A had few pyramidal neuronal shrinkage, some pyknosis, prominent vacuolations across the three layers of the hippocampus, and vascular congestion; 2B show petechial haemorrhage with neuronal hypertrophy with signs of oedema and some neuronal polymorphism; 2C has slight palely stained neuropil with mild hypotrophy; 2D has prominent neuronal shrinkage across the three layers and mild signs of lesion in the neuropil. Cellular damage may occur by enzymatic digestion and leakage of cellular contents presenting with enlarged cell size, transformed nuclei with pkynosis, karyorrhexis and karyolysis [34]. Neuronal shrinkage common in most of the plates is an early and easily recognizable indicator of neuronal degeneration in the hippocampus [35]. The large pyramidal cells in area CA1 are exceptionally sensitive to oxygen deprivation and die after few minutes without a supply of fresh arterial blood, and pathologists call the area CA1 Sommer’s sector. The hippocampal pyramidal cells are among the first to be affected in a variety of conditions that lead to loss of memory and intellectual functions, including Alzheimer’s disease [16]. Brain oedema is largely a result of astrocyte swelling, which in turn is caused by astrocytic uptake of NH4+ regarded initially as a neuroprotective mechanism; it also drives the synthesis of glutamine from glutamate by glutamine synthetase, resulting in changes in transmitter homeostasis that may contribute to behavioural symptoms. The accumulation of glutamine in astrocytes also leads to osmotic stress with astrocyte swelling and progressive cytotoxic oedema [36].GFAP expression is a sensitive and reliable marker that labels most; if not all, reactive astrocytes that are responding to CNS injuries [37]. The neuro-inflammatory marker reveals that in Figure 3 (A to D) there was correlation between GFAP expression and severity of P.berghei infectivity, parasite load as well as H&E histomorphology thus; 3A and 3B showed the most severe reactive astrogliosis; 3C was mild to moderate immunoreactivity, whereas 3D had very mild or the least detectable GFAP expression. NL has been reported to possess anti-inflammatory property [38] and [39] have reported on how polyphenolic antioxidants from vegetables reduce or block neuronal death occurring in the pathophysiology of some diseases. Treatment of infected mice with N. latifolia aqueous leaf extract reduced lipid peroxidation and oxidative stress, by probably improving antioxidant defense [27].In conclusion, ethanolic leaf extract of N. latifolia like 5 mg Artemether/Lumefantrine (Coartem®) per kg body weight in mice clears parasitemia, offers moderate neuroprotection to the hippocampus of P. berghei-infected mice at a dose dependent level, and down-regulates the expression of neuro-inflammatory marker glial fibrillary acidic protein. Further studies to similarly challenge the fractions of the N. latifolia extract and develop pure compound is necessary.
ACKNOWLEDGEMENTS
- Mr. Malachy Nsikan, of the Department of Pharmacology and Toxicology, University of Uyo, Uyo, Nigeria for his technical assistance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML