-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2017; 6(1): 1-8
doi:10.5923/j.ijbcs.20170601.01

A Comparison of Perceived and Actual; Students’ Learning Difficulties in Physical Chemistry
Francis A. Adesoji 1, Nathaniel Ayodeji Omilani 2, Sakin Olanrewaju Dada 1
1Department of Teacher Education, Faculty of Education, University of Ibadan, Ibadan, Nigeria
2Integrated Science Department, Federal College of Education, Abeokuta, Nigeria
Correspondence to: Nathaniel Ayodeji Omilani , Integrated Science Department, Federal College of Education, Abeokuta, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Having established from researches that students experience learning difficulties in physical chemistry (electrolysis) and that those researches have not been able to find an end to the learning difficulties known through the use of questionnaires, the study made a comparison of perception and actual students’ learning difficulties in physical chemistry (electrolysis). Two hundred chemistry students were selected purposively from thirteen Senior Secondary School Three classes in Ido local government of Oyo State, Nigeria. Three research question and a hypothesis were raised. The instruments used for the collection of data were Students’ Learning Difficulty in Physical Chemistry Questionnaire (r = 0.98) and Physical Chemistry Learning Difficulty Diagnostic Test (r = 0.87). The data collected were analysed using simple percentage and chi-square analysis. The result showed that many areas of physical chemistry where students perceived no difficulty were actually difficult for them to solve. It was recommended among other things, that students’ perceived difficulty should not be used as the basis for understanding the learning difficulties in electrolysis.
Keywords: Comparison, Perceived learning difficulty, Actual learning difficulty, Physical Chemistry
Cite this paper: Francis A. Adesoji , Nathaniel Ayodeji Omilani , Sakin Olanrewaju Dada , A Comparison of Perceived and Actual; Students’ Learning Difficulties in Physical Chemistry, International Journal of Brain and Cognitive Sciences, Vol. 6 No. 1, 2017, pp. 1-8. doi: 10.5923/j.ijbcs.20170601.01.
Article Outline
1. Introduction
- Students’ academic performance in any subject is an important index for measuring the effectiveness of teaching/learning and the extent to which the intended objectives of the subjects are being achieved (Okunloye and Awowale, 2011). Any malfunction during the teaching/learning process can result in students’ poor performance. Students perform poorly in Chemistry, a central science subject. This situation is evident in the frequent poor achievement of Nigerian students as measured by their cognitive achievement in the May/June and November/December Senior School Certificate Examination (SSCE) for Chemistry. For instance, the West African Senior School Certificate Examinations (WASSCE) from 2001 to 2012 indicated that 50% of students who sat for the examinations were able to obtain at least a credit pass in the subject in the years 2005, 2006 and 2010 while the remaining years recorded below 50% credit pass.The inability to effectively grasp chemistry concepts leaves students with no other option than to engage in rote memorization. For many students, chemistry is seen as a difficult, complex and abstract subject that requires special intellectual talent and too much effort to understand (Cardellini, 2012). Also, because students have a poor grasp of concepts in Chemistry at the secondary school level, it becomes a great challenge for them to study chemistry- related courses at tertiary institutions. The investigation by Yusuf and Ali (2012) revealed that environmental challenges hinder many students from getting interested in chemistry courses in universities. Consequently, students are faced with greater challenges which may result in failing and retaking failed courses or withdrawal from school. Put succinctly, students are faced with learning difficulties in chemistry.To abrogate or curtail this dreaded situation, educational researchers, teachers, educational providers and many other agencies have tried, using questionnaires, to seek the students’ perception in order to ascertain the areas and causes of students’ learning difficulties in chemistry and experiment with new teaching methods. Understanding students’ difficulties in chemistry depends on students’ perception of the content of chemistry (Gulacar and Bowman, 2014). The knowledge of secondary school students’ perception of chemistry is useful for designing strategies for uplifting and maintaining positive student attitudes (Autida, 2012). The end users of every instruction in the class are the students. In spite of the efforts made by the educational researchers, teachers, educational providers and many other agencies, students’ achievement in chemistry has been poor and unsatisfactory years after year (Nbina and Auwiri, 2014; Udoh, 2012). The teaching and learning of chemistry in secondary schools is not at its best.Despite the numerous findings by quite a number of researchers on difficulties students’ encounter when learning Chemistry, students’ learning difficulties in Chemistry still persist. Therefore, it becomes imperative to look into what really transpired in the researches conducted in order to proffer solutions aimed at ending students’ learning difficulties in chemistry. Previous researches conducted to investigate the causes and areas of learning difficulties in chemistry available to the researcher (Agogo and Onda, 2014; Childs, and Sheehan, 2009; Gafoor, and Shilna, 2013; Gongden, Gongden and Lohidip, 2011; Jimoh, 2005; Kazembe and Musarandega, 2012; Onuekwusi, 2015; Soubhi, Touri, Lima, Knouzi, Talbi, and Kasour, 2014), revealed that efforts have primarily been concentrated on identifying identified students’ difficulties in chemistry using questionnaires in which students were required to rate chemistry topics on a scale of “very difficult”, “difficult”, and “not difficult”.Only a handful of studies on students’ learning difficulties actually administered a diagnostic test on chemistry content and compared it with the actual perception. It should be noted that the students’ perception, which is a mental view about their learning difficulties, is constructed as a result of their social experiences developed through interaction with their environment (e.g. school, home) or the influence of peers, teachers, parents, siblings or mass media. The students’ perception could also have been influenced by past experiences, beliefs, attitudes, conceptions and misconceptions. The students might have been painting wrong pictures which did not exist about their learning difficulties in chemistry.Perhaps the learning difficulties of students in Chemistry might have lingered or unresolved because the common approach of assessing the difficulty, often from the affective domain of feeling and perception. This is approach is often not well integrated and articulated with a diagnosis of the difficulty by administering a test to the learner and analysing the actual area of difficulty. This dependence on students’ perceived difficulty without considering their actual difficulty for decision making can also be explained through gap analysis model developed by Parasuraman, Zeithaml and Berry (1985) which has been used in business and economics as a tool that helps an organization to compare its actual performance with its potential performance. In applying this to education, Anthony (2012) defined gap analysis as the determination of the difference between current knowledge/practice and current evidence based practice. In education, the quality of teaching which is delivered to students is a function of the gap between perceived learning and actual learning. The gap can be evident in the cognitive, affective and psychomotor domains. Gap analysis is not an end in itself, but a catalyst to shedding light for improvement on weak areas and reinforcement of strong areas.The continuous lingering problem of students’ learning difficulties in Chemistry might have persisted because conclusions were drawn and recommendations were given based on the findings got from students’ perceptions only which might have been erroneously provided. This is because recommendations made were not proactive due to incorrect perceptions. Therefore, there is a need to make a comparison on perception and actual students’ learning difficulties to aid provision of proactive recommendations.Moreover, studies on difficult topics in Chemistry indicated that not all Chemistry topics were perceived as difficult. This makes it imperative to ascertain the actual branches of Chemistry where the topics posing learning difficulties to students are embedded. The findings of Gongden, Gongden and Lohdip (2011), Jimoh (2005), Onuekwusi (2005) and Sai (2010) revealed that students perceived topics in physical chemistry as being difficult to learn while electrochemistry (electrolysis) as an aspect of chemistry was asserted by De Jong and Treagust (2002) to be the most difficult chemistry topic taught and learnt in secondary school.Bain, Moon, Mack and Towns (2014), Becker and Towns (2012), Shadreck (2013), Sokrat, Tamani, Moutaabbid and Radid (2014), Taha, Hashim, Ismail, Jusoff and Yin (2014), and Turanyi and Toth (2013) conducted researches on physical chemistry or topics under physical chemistry. They found out the following amongst others as indicators of students’ learning difficulties which consequently led to poor students’ achievement in physical chemistry: students’ poor mathematical ability, poor physics background, poor understanding of the particular nature of matter, poor problem solving skills, mixing up of concepts and the abstract nature of chemistry. Others include a lack of students’ motivation and poor students’ attitude towards physical chemistry (students-related factors), teacher-centered pedagogies (teacher-related factor), insufficient teaching and learning resources (environmental-related factor). For effective scrutiny in the study, only subject-related factors will be considered. Therefore, this research made a comparison on perception and actual students’ learning difficulties in physical chemistry (electrolysis in particular) taking into consideration subject factors in relation to students’ ability.
2. Statement of the Problem
- Chemistry has been recognized a central science subject in secondary in Nigeria and therefore it is important to the development of Nigeria as a nation. The incessant poor performance of students in Chemistry especially at national and international examinations needs proactive attention. The high rate of failure has hampered students from furthering their education in science leading to a gradual fading-off of science education because at least a credit pass in Chemistry is needed by students seeking admission into tertiary institutions to study science-related courses. Consequently, the development of the country is at stake. Many researches have indicated that students have difficulty in learning physical chemistry, especially, electrolysis. In spite of the efforts of educational researchers to end the endemic students’ learning difficulties in Chemistry, the problem persists. In most cases, the perception of students were sought without using diagnostic test to ascertain whether learning difficulties existed in the acclaimed areas. The questionnaire, which has been the frequently used instrument to ascertain students’ learning difficulties in Chemistry, sometimes does not give the true picture of a given situation because it can be affected by the students’ past experiences and surroundings. Many a times, recommendations might have been based on erroneous students’ perceptions. This could have led to the persistence of students’ learning difficulties in Chemistry. It is thus necessary to make a comparison between the students’ perception of their learning difficulties in physical chemistry (obtainable with a questionnaire) and the actual learning difficulties they exhibit (obtainable with the aid of a diagnostic test).Research Questions1. What are the students’ perceived learning difficulties in physical chemistry?2. What are the actual learning difficulties in physical chemistry exhibited by students?3. Are the students’ perceived learning difficulties in physical chemistry actually exhibited by the students?Research HypothesisThere is no significant difference between the students’ perceived and actual learning difficulties in physical chemistry.
3. Methodology
- Research design: This study adopted the survey design using the ex-post facto type. This design is chosen because the researcher has no control over the independent variables and their manipulation has already occurred.Sampling: The sample of the study is made up of Senior Secondary School 3 (SSS 3) Chemistry students who have been taught electrolysis. They were selected from thirteen public and private secondary schools in Ido local government area. Purposive sampling was used to select two hundred students.Instruments: The instruments used were: Students’ Perceived Learning Difficulty in Physical Chemistry Questionnaire (SPLDPCQ) and Physical Chemistry Learning Difficulty Diagnostic Test (PCLDDT) with reliability coefficients of 0.98 and 0.87 respectively. Some questions in PCLDDT were adapted from Sai (2010). The instruments were validated by two Chemistry teachers, two experts in test and measurement and an expert in science education. The final design of PCLDDT was administered to thirty students who were not part of the sample of study. The SPLDPCQ, which was used to determine students’ perceived learning difficulties in physical chemistry, has two sections – A and B. Section A provides information on students’ biodata which consists of students’ name of school, class, gender and age. Section B consists of nine statements on perceived learning difficulty in electrolysis which students were to respond to based on a four-point Likert ordinal scale of strongly agree (SA), agree (A), disagree (D), and strongly disagree (SD). The PCLDDT, which was used to determine students’ actual learning difficulties in physical Chemistry, also has two sections. Section A covers students’ biodata (students’ name of school, class, gender and age). Section B consists of essay based test items that cover all the nine areas of the perception questionnaire. Students were required to solve the problems and show their answers. Administration of Instruments: The researcher personally administered the PCLDDT and SLDPCQ respectively on the same day to the sampled students and collected answered research instruments immediately before leaving the schools.Method of Data Analysis: The data collected were analysed using percentage and chi square analysis.
4. Results
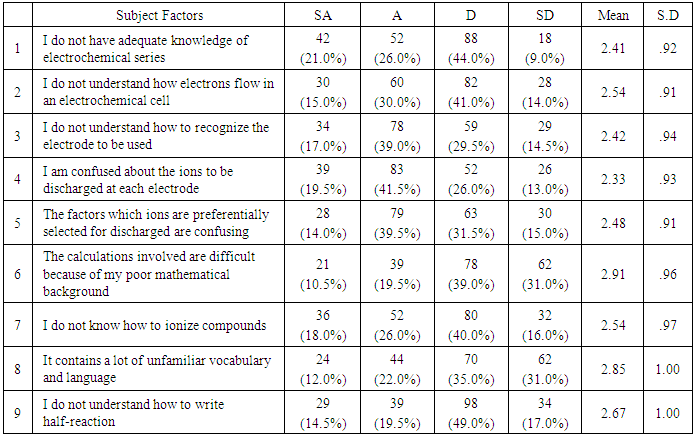
- The research questions are answered below. It was decided that for an item to be classified as showing wide- spread difficulty for students, it would be experienced by at least 50% of the students.Research question 1: What are the students’ perceived learning difficulties in physical chemistry?Students’ perceived difficulties on content areas of physical chemistry were obtained by means of the Students Learning Difficulty in Physical Chemistry Questionnaire (SLDPCQ). Table 1 shows that: (1) students thought they understood how electrons flow in an electrochemical cell; (2) the calculations involved were not difficult because students did not have poor mathematical background; students knew how to ionize compounds; (3) physical chemistry (electrolysis) did not contain a lot of unfamiliar vocabulary and language and that students understood how to write half-reaction. However, Table 1 reveals that students did not have adequate knowledge of electrochemical series; students did not understand how to recognize electrode to be used; students were confused about ions to be discharged by each electrode; and the factors which determines how ions are preferentially selected for discharge were confusing. With a grand mean of 2.57, subject factors were not generally perceived as causes of learning difficulty in physical chemistry.
|
|
|
|
5. Discussion of Results
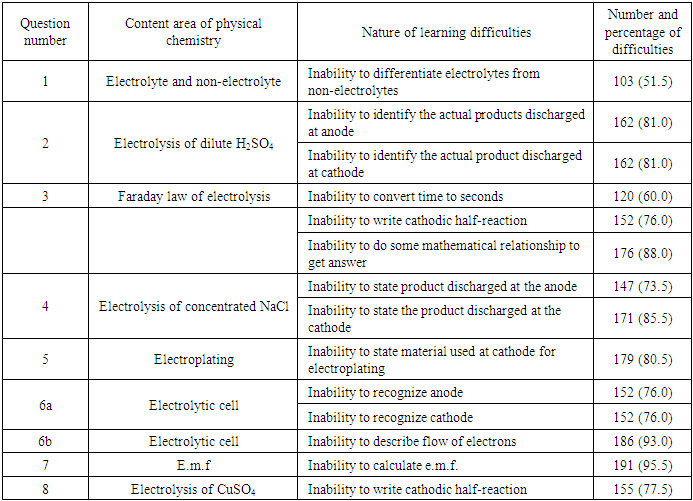
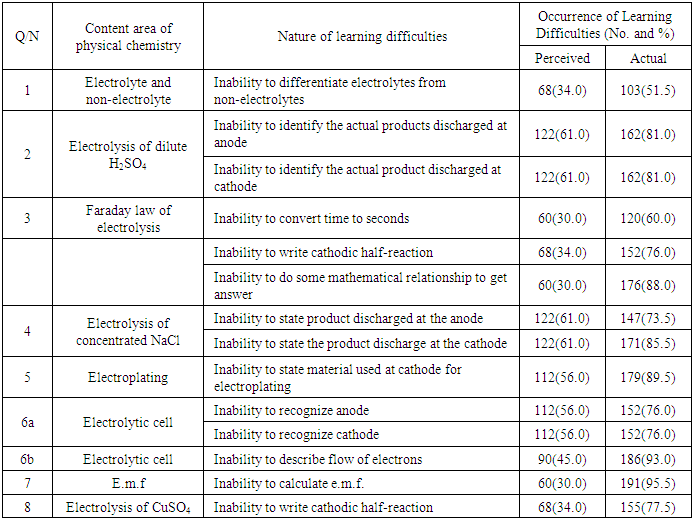
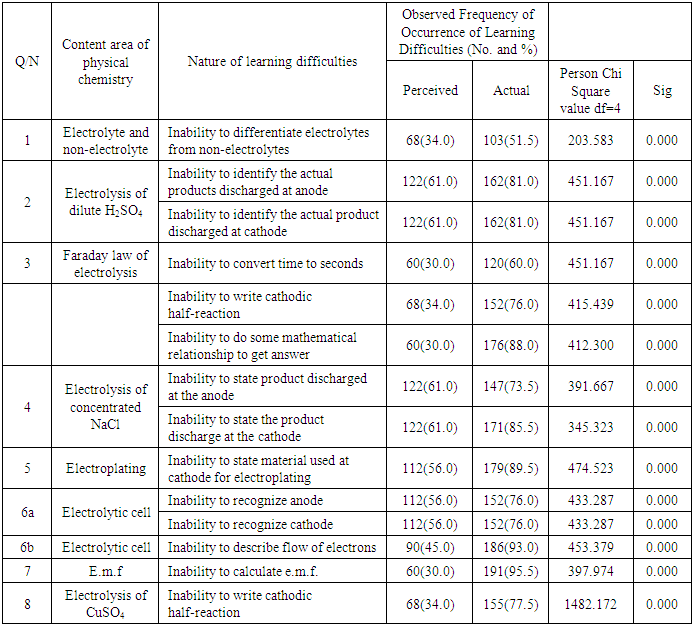
- The results showed that there was no disparity in a few perceived and actual students’ learning difficulties in physical chemistry. Students perceived that they were confused about ions to be discharged at each electrode and that factors determining how ions are preferentially selected for discharge were confusing. The perceptions were evident in their inability to identify products obtained at each electrode in the electrolysis of dilute H2SO4 and concentrated NaCl. The perception that students did not know how to recognize electrode was evident in their inability to recognize cathode and anode in an electrolytic cell. This is in line with the finding of Grant, Malloy and Murphy (2009) that there was no difference in students’ perception and performance for their presentation skills. Also, Shapiro and Dummer (1998) found out that there was a positive relationship between perceived and actual basketball competence for the individual skills of push pass for accuracy, jump and reach, speed dribble, and free-throw shooting.However, there was a wide discrepancy between perceived and actual students’ learning difficulties in physical chemistry. Many students perceived that they understood how electrons flow in an electrolytic cell but they were unable to describe the flow of electrons in an electrolytic cell in the diagnostic test. Also, many students perceived that calculations in physical chemistry (electrolysis) are not difficult because they have good mathematical ability but they were unable to do so in the real test. For example many were unable to convert time given to: seconds; calculate the mass of gold deposited and to calculate e.m.f. of a cell due to poor mathematical ability exhibited. This shows that during the process of teaching and learning, the false perception of their ability to solve problems relating to Physical Chemistry may hinder many of them from benefiting from instruction, correction of assignment and revision classes. Furthermore in the teaching-learning situation, the teacher often asks students this question after instruction: “Do you understand?” Most of the time, the students respond with a chorus, “Yes”. It is important to observe that amidst students’ chorus affirming they understand, are voices of students who respond based on perception and not actual ability. The result of this study showed that students with poor mathematical background find it difficult to solve calculations in Physical Chemistry. This is in line with the finding of Eze-Odurukwe (2014) that poor mathematical background is the leading cause of inability to solve chemical arithmetic problem. This is also in line with Aje’s (2005) finding that significant positive relationship existed between students’ performance in mathematical achievement test and their performance in a stoichiometry test. In addition, students perceived that they understood how to write half-reaction but they had difficulty in writing cathodic half-reaction of gold and copper. The results of disparity between the perceived and actual students’ learning difficulties in chemistry obtained in this study were similar to some results got by some researchers who carried out researches on comparison of perception and actual situation. The findings of Grant, Malloy and Murphy (2009) indicated some differences in the students’ perception of their word processing skill and actual performance and a significant difference in perception and performance for students’ spreadsheet skills. Also, the findings of Sangstar, Anderson and O’Hara (2012) revealed that student-teachers’ level of linguistic knowledge as measured by the instrument employed were generally low contrasting with their own more positive perceptions of their competence. Besides, findings of Sitzmann, Ely, Brown and Bauer (2010) indicated that there was zero correlation between self-reported knowledge gain (perceived learning) and actual knowledge.The findings of the study shows that in many areas of physical chemistry where students perceived no learning difficulty; they actually had learning difficulty in those aspects of physical Chemistry. It is now evident why researches on students’ learning difficulties in chemistry have not been able to tackle the endemic problem among other things. This is because recommendations have been given based on a lot of erroneous findings from a number of previous researches.
6. Recommendations
- The following recommendations were made based on the results got from the study.v Students’ perceptions should not be used alone to recommend proactive solutions to lingering students’ learning difficulties in Chemistry.v The same research should be done on other topics/aspects of Chemistry where students are also experiencing learning difficulties.v Teachers should educate students on how to give correct perceptions of situations.v All pre-requisite concepts to physical chemistry (electrolysis in particular) should be well taught before teaching physical chemistry so as to eradicate learning difficulty.v The Ministry of Education, curriculum developers and teacher educators should include a systematic training of teachers in diagnostic testing and remedial teaching approaches for both pre-service as well as in-service teachers in their developmental programme.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML