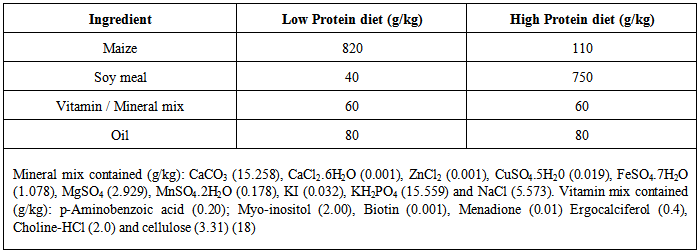
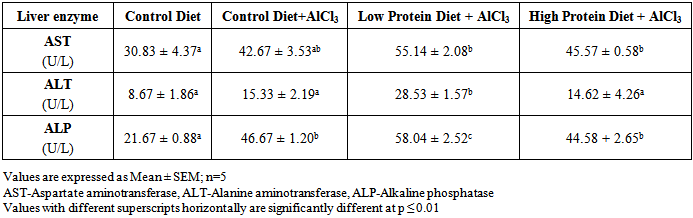
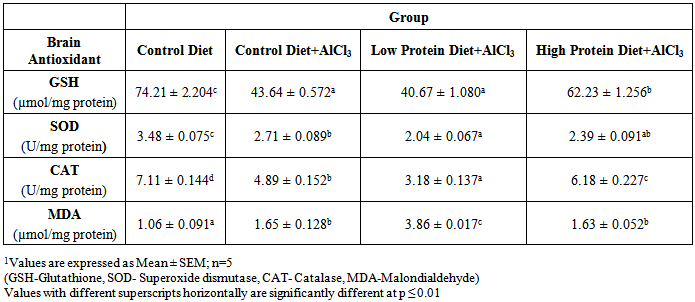
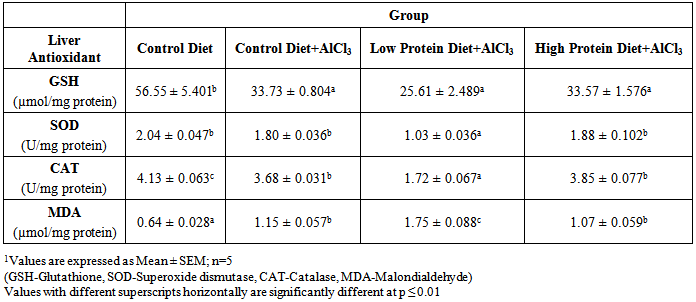
| [1] | De Onis, M. and Blössner, M. (1997). WHO Global Database on Child Growth and Malnutrition; World Health Organization: Geneva, Switzerland; p. 74. |
| [2] | Galler, J. R. (2001). Children and famine: long term effects on behavioural development. Ambulatory Child Health; 7:85–95. |
| [3] | Ebuehi, O.A.T. (2012). Nutrients and neurotransmitters: The chemicals of life in the brain. 10th Inaugural Lecture series, delivered at Main Auditorium, University of Lagos, University of Lagos Press, Lagos, Nigeria, March 21, 96pp. |
| [4] | Ebuehi, O.A.T., Salawu, O.M. and Akinwande, A.I. (1999). Effect of post-weaning protein malnutrition on organ growth, protein malnutrition and lipid composition in rats. Nig. Journal of Medical Research 4: 84-88. |
| [5] | Nayak, P. and Chatterjee, A. K. (2003). Dietary protein restriction causes modification in aluminum-induced alteration in glutamate and GABA system of rat brain. BMC Neurosci. 4, 4–11. |
| [6] | Winick, M. and Rosso, P. (1969). The effect of severe early malnutrition on cellular growth of the human brain. Pediatr. Res.; 3:181– 4. |
| [7] | Ebuehi, O.A.T and Akande, G.A. (2009). Effect of zinc deficiency on memory, oxidative stress and blood chemistry in rats. Int. J. Biol. Chem. Sci. 3(3): 513-523. |
| [8] | Becaria, A., Campbell, A. and Bondy, S. C. (2002). Aluminum as a toxicant. Toxicol. Ind. Health. 18, 309–320. |
| [9] | Turgut, G., Kaptanoglu, B., Enli, Y. and Genc, O. (2004). Effect of chronic aluminium administration on blood and liver iron related parameters in mice .Yonsei Med. J. 45(1), 135-149. |
| [10] | Zatta, P., Lucchini, R., van Rensburg, S. J. and Taylor. A. (2003). The role of metals in neurodegenerative processes: aluminum, manganese, and zinc. Brain Res. Bull. 62, 15–28. |
| [11] | Yokel, R. A. (2000). The toxicology of aluminum in the brain: a review. Neurotoxicology 21, 813–828. |
| [12] | Cannata, J.B. Serrano, M. and Fernandez, S.I. (1998). Minimizing the risk of oral aluminium exposure in chronic renal failure. Contrib Nephrol. 71, 81-9. |
| [13] | Yokel, R. A. and McNamara, P. J. (2001). Aluminium toxicokinetics: an updated minireview. Pharmacol. Toxicol. 88, 159–167. |
| [14] | Abbasali, K.M., Zhila, T. and Farshad, N. (2005). Developmental toxicity of Aluminum from high doses of AlCl3 in mice. J. Applied Res., 5: 575-579. |
| [15] | ATSDR (Agency for Toxic Substances and Disease Registry) (1999). Toxicological profile for aluminium (update). Atlanta, GA, U. S. Department of Health and Human Services, Public Health Service (TP-91/01). |
| [16] | Exley, C. (2004). The pro-oxidant activity of aluminum. Free Radic. Biol. Med. 36, 380–387. |
| [17] | Roskams, AJ and Connor, JR (1990). Aluminium access to the brain: a role or transferring and its receptor. Proc. Natl. Sci USA, 87,9024-9027 |
| [18] | Adelusi, S.A. and Olowookere, J.O. (1985). A rapid method of induction of post-natal protein-energy malnutrition (PEM) in laboratory animals Nig. J. Appl. Sci., 3: 171-174. |
| [19] | Gowri Shankar, N. L, Manavalan, R., Venkappayya, D. et al. (2008). Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl(4)-induced oxidative damage in rats. Food Chem Toxicol 46: 3182-185. |
| [20] | AOAC, (2005). Official Methods of Analysis of AOAC International. p. 42-1-42-2. In T.R.Mulvaney (ed.) AOAC International, Arlington, VA. |
| [21] | Halas, E.S., Eberhardt, M.J., Diers, M.A. and Sandstead, H.H. (1983). Learning and memory impairment in adult rats due to severe zinc deficiency during lactation. Physiol. Behav., 30: 371-381. |
| [22] | Varshney, R. and Kale, R.K. (1990). Effect of calmodulin antagonists on radiation induced lipid peroxidation in microsomes. Int. J. Radiat. Biol., 58:733–743 |
| [23] | Jollow, D.J., Mitchell, J.R., Zampaglione, N. and Gillete, J.R. (1974). Bromobenzene induced liver necrosis: protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology, 11, 151-169. |
| [24] | Sinha, K.A. (1971). Colorimetric assay of catalase. Anal Biochem., 47: 389-394. |
| [25] | Misra, A. and Fridovich, H. (1972). Determination of the level of superoxide dismutase in whole blood. Yale Univ. Press New Haven, pp: 101-109. |
| [26] | Julka, D., Sandhir, R. and Gill, K. D. (1996). Altered cholinergic metabolism in rat CNS following aluminum exposure: implications on learning performance. J. Neurochem. 65, 2157–2164. |
| [27] | Sadasivudu, B. and Murthy, C.R.K (1978). Effect of ammonia on monoamine oxidase and enzymes of GABA metabolism in mouse brain. Arch. Int. Physiol. Biochem. 86, 67-82 |
| [28] | Abu-Taweel, G.M., Ajarem, J.S. and Ahmad, M. (2012). Neurobehavioural toxic effect of perinatal oral exposure of aluminium on the development motor reflexes, learning, memory and brain neurotransmitters of mice offspring. Pharmacol. Biochem Behav. 101 (1): 49-56. |
| [29] | Yousef, M. I. (2004). Aluminum-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acid. Toxicol. 199, 47-57. |
| [30] | Nikolova, P., Softova, E., Kavaldzhieva, B. et al. (1994). The functional and morphological changes in the liver and kidneys of white rats treated with aluminum. Eksp Med Morfol 32: 52-61. |
| [31] | Gowri Shankar, N. L, Manavalan, R., Venkappayya, D. et al. (2008). Hepatoprotective and antioxidant effects of Commiphora berryi (Arn) Engl bark extract against CCl(4)-induced oxidative damage in rats. Food Chem Toxicol 46: 3182-185. |
| [32] | Bogdanovic, M., Janeva, A.B. and Bulat, P. (2008). Histopathological changes in rat liver after a single high dose of aluminium. Arh Hig Rada Toksikol 59: 97-101. |
| [33] | Sinha, U. S, Kapoor, A. K. and Singh, A.K. (2005). Histopathological changes in cases of aluminium phosphide poisoning. Indian J Pathol Microbiol 48: 177-80. |
| [34] | Singhal, R. L. and Merali, Z. (1977). Biochemical toxicity of cadmium. In: Mennear JH. ed. Cadmium toxicity. Marcel Dekker; pp. 61-112. |
| [35] | Rahman, M. F., Siddiqui, M. K. and Jamil, K. (2000). Acid and alkaline phosphatase activities in a novel phosphorothionate (RPR-11) treated male and female rats. Evidence of dose and time dependent response. Drug Chem Toxicol 23: 497-509. |
| [36] | El-Demerdash, F.M., Yousef, M.I., Kedwany, F.S. and Baghdadi, H.H. (2004). Role of α-tocopherol and β-carotene in ameliorating the fenvalerate-induced changes in oxidative stress, hematobiochemical parameters and semen quality of male rats. J. Environ. Sci. Health Bull., 39: 443-459. |
| [37] | Mansour, S., Alan, S. and Norman, B.R. (2006). Aluminum-induced injury to kidney proximal effects on markers of oxidative damage. J. Trace Elem. Med. Biol., 19: 267-273. |
| [38] | Moumen, R., Ait-Oukhatar, N., Bureau, F., Fleury, C., Boublé, D., Arhan, P., Neuville, D. and Viader, F. (2001). Aluminium increases xanthine oxidase activity and disturbs antioxidant status in the rat. J. Trace Elem. Med. Biol. 15, 89–93. |
| [39] | Anane, R. and Creppy, E.E. (2001). Lipid peroxidation as a pathway to aluminum cytotoxicity in human skin fibroblast cultures: Prevention by superoxide dismutase plus catalase and vitamins E and C. Hum. Exp. Toxicol., 20: 477-481. |
| [40] | Nehru, B. and Anand, P. (2005). Oxidative damage following chronic aluminium exposure in adult and pup rat brains. J. Trace Elem. Med. Biol. 19, 203–208. |
| [41] | Sánchez-Iglesias, S., Méndez-Alvarez, E., Iglesias-González, J., Muñoz-Patiño, A., Sánchez-Sellero, I., Labandeira-García, J. L. and Soto-Otero R. (2009). Brain oxidative stress and selective behaviour of aluminium in specific areas of rat brain: potential effects in a 6-OHDAinduced model of Parkinson's disease. J. Neurochem. 109, 879–88. |
| [42] | Harris, W. R., Wang, Z. and Hamada Y. Z. (2003). Competition between transferrin and the serum ligands citrate and phosphate for the binding of aluminum. Inorg. Chem. 42, 3262–3273. |

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML