-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2015; 4(2): 28-32
doi:10.5923/j.ijbcs.20150402.02
Topography of Deep Cerebellar Nuclei of the African Giant Pouched Rat (Cricetomys gambianus)
Byanet Obadiah
Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Nigeria
Correspondence to: Byanet Obadiah, Department of Veterinary Anatomy, College of Veterinary Medicine, University of Agriculture, Makurdi, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
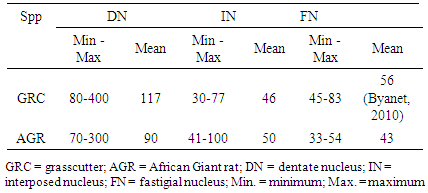
The deep cerebellar nuclei form the major structures that cerebellum communicates with other brain components to execute its functional role of coordination of voluntary movement, posture and balancing. The major objective of this study was to identify as well as relating the deep cerebellar nuclei to the zonal distributions in the cerebellum. A histomorphological analysis was conducted on twelve cerebella of African giant pouched rats. Three nuclei were identified to be associated with the corresponding zone of the cortex; the dentate were noted in the bilateral cerebellar hemispheres, interpose nuclei were found in the paravermis and the fastigial nuclei in the central vermis. The dentate nuclei were the largest of the three, average 90 µm, composed of multipolar, bipolar neurons and some characteristic cellular bands in form of a folded bag shaped cells. The interposed nuclei were next in size to dentate, averaged 50 µm composed mainly of multipolar and bipolar neurones. The smallest was fastigial nuclei, averaged 43 µm and composed of small and medium-sized bipolar neurones. In conclusion, the relatively well-developed deeper cerebellar nuclei, particularly the dentate nucleus in this species may be correlated with the animals’ ability to use their extremities in movement and in reaching and grasping of grasses and other food materials in the wild and / or captivity.
Keywords: Morphology, Deep cerebellar nuclei, C. gambianus
Cite this paper: Byanet Obadiah, Topography of Deep Cerebellar Nuclei of the African Giant Pouched Rat (Cricetomys gambianus), International Journal of Brain and Cognitive Sciences, Vol. 4 No. 2, 2015, pp. 28-32. doi: 10.5923/j.ijbcs.20150402.02.
Article Outline
1. Introduction
- The African giant pouched rat (Cricetomys gambianus) is one of Africa's largest rodents found mostly in savannas, around the edges of forests and within the human settlements. Their burrows can be complex are commonly seen inside termite mounds and at the base of trees. They are nocturnal animals and usually lead solitary lives and forage alone [1].The search for alternative animal protein source has popularized attempts to domesticate and breed wild rodents such as African giant pouched rat (AGR) in African sub region (National Research Council [1]. The AGR has almost the same capacity to produce animal protein comparable to those produce by rabbits in tropical countries [2]. The basic biology, particularly nervous system among others is considered as the research need for their effective domestication. Cerebellum is an important part of the brain that is responsible for coordination of complex movements [3]. The early anatomical studies proposed a concept for morphological organization of the cerebellum based on the efferent relationship between the cerebellar cortex and the deep cerebellar nuclei (DCN) of the cat, rabbit and monkey [4]. Furthermore, these authors found that cerebellum was organized into zones; the vermis, paravermis and lateral hemispheres cortex. It has been shown that the mouse DCN comprises of three distinct nuclei (dentate, interposed and fastigial) and each has neurones of various sizes [5]. Also, each pair of DCN is associated with a corresponding zone of the cerebellar cortex, such that the dentate nuclei are placed in the lateral hemispheres; the interposed nuclei are located in the paravermis and the fastigial nuclei in the central vermis [6]. These structural relationships are generally maintained in the neuronal connections between the nuclei and associated cerebellar cortex [5]. The dentate nuclei (DN) are found to be the largest and laterally placed on both sides of the cerebellar hemispheres in man and animals [7]. In transverse section, some DN neurons appear as convoluted band of gray matter, with folded bag -like shaped and opening or hilus directed medially [8]. In addition, the DN neurones are composed mainly of large multipolar cell types with branching dendrites [9]. Comparative studies revealed that DN is large in great apes and humans relative to the other cerebellar nuclei, due to its relative expansion in size on the ventral region [10-11]. This implies that the non-motor (ventral) region of the dentate is of importance in great apes and humans [12]. Generally, cerebellar nuclei control the non-motor such as cognitive events and motor activity that concerned with voluntary movements of the extremities, including reaching and grasping [13-15]. With functional magnetic resonance imaging, the fastigial nucleus (FN) and globose / emboliform are seen to be thin and located close to the gray matter of lobules VIII and IX of the cerebellar cranial lobe [11]. The globose and emboliform nuclei in mammals are collectively referred to as nucleus interpositus or interposed nucleus (IN), particularly in lower mammals [7, 9]. Emboliform nucleus is a wedge-shaped cell mass situated close to the hilus of the DN, and composed of cells similar to those found in the DN [6]. In lower mammals, the IN is said to be divided into two parts: the anterior interposed nucleus, considered the homologue of the emboliform nucleus and the posterior interposed nucleus, which is homologous to the globose nucleus [9]. In the whale, the largest of the three cerebellar nuclei is the IN, and not the DN. It has been observed that IN aids in getting sensory information from bodily receptors [16], and has been particularly active during the modulation of various reflexes and sensory feedback, such as eye blinks responses [17]. The FN is also divided into rostral and caudal components [14]; with the rostral portion involvs in the control of somatic musculature, head orientation and eye-gaze shifts, while, the caudal portion plays key roles in saccade generation and smooth pursuit [18].The structures and functions of DCN have been documented in some rodents (rats, guinea pig, chinchilla), domestic animals (dog, cat, pig, cattle, horse, etc.) and humans. The quantitative neuroanatomical investigations of these nuclei in these animals are becoming more advanced, but there is a paucity of such information in most African rodents such as African giant rat. The aim of this study was to quantitatively investigate the basic structure of DCN in the African giant pouched rat and relate the findings to functions in the wild / or captivity.
2. Materials and Methods
2.1. Sources of Animals and Study Location
- A total of twelve African giant rats (AGRs) were used in the study. The animals were trapped alive in the wild in Zaria (11°10/N, 07° 38/E), Northern Nigeria and reared under laboratory conditions in the Department of Veterinary Physiology and Pharmacology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria. They animals were transferred in standard laboratory cages to the nearby Veterinary Anatomy Research Laboratory, of the same university, where the research was conducted.
2.2. Brain Extraction and Fixation
- The rats were anesthetised with 10-25 mg/kg IM ketamine Hcl. The head of each was decapitated at the atlanto-axial joint using a small sharp knife and forceps and the head skinned and stripped of all muscles. The brain was then extracted from the skull [6] with some modifications due to the peculiarity of the rodents. The brains were grossly examined for pathological lesions and were found to be apparently normal. The cerebellum was separated from the brainstem through the three cerebellar peduncles and fixed in 10% formalin.
2.3. Histological Findings
- The fixed cerebellar tissues were cut into blocks and dehydrated through a series of graded alcohol (70%, 80%, 90%, 95% and 100%), cleared in xylene and infiltrated with molten paraffin wax. Sections of 6 μm thick were cut from the embedded tissue using Jung Rotary Microtome (model 42339). The cresyl violet staining was carried out as described [19]. Briefly; to 0.5% Cresyl Echt Violet (Cresyl Echt violat acetate, 0.5g) was added 100ml of distle water and mixed. The paraffin sections (6 µm) were deparaffinized and hydrated with distilled water. The sections were then placed in cresyl violet solution for 2 minutes and washed in water before dehydrated with alcohol. The sections were then cleared in xylene and covered with cover slips. They were then mounted with permount and viewed under light binocular microscope (Olympus Binocular Microscope). Micro-photographic images were viewed at various magnifications (100x, 200x and 400x) captured at 400x after colour adjustment using Digital Microscopic Eyepiece (ScopePhoto 3.0; Version 3.0 of 2003-2007). The processing of the captured microscopic images was done using Motic Images Plus 2.0 ML Multi language version http://www.motic.com (Motic China Group Co., Ltd of 2001-2006) at the resolution, 640 X 480 JPG pixels. The images were then transferred to the Microsoft Office Power Point where they were labeled, and legends were written before finally saved in Microsoft Office Word. The sizes of the three DCN were measured (µm) and tabulated the compared with the previous data on the grasscutter (Byanet, 2009).
3. Results
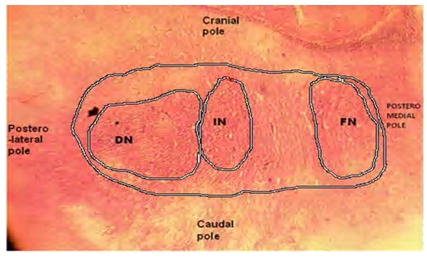
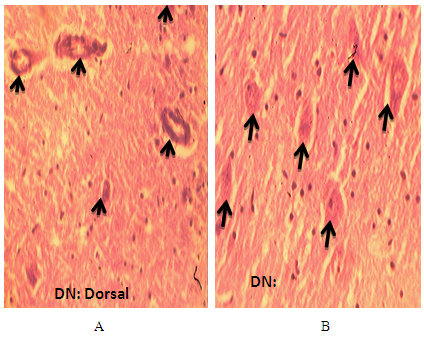
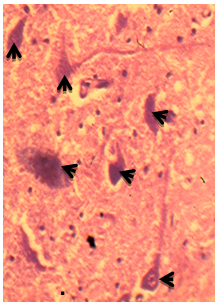
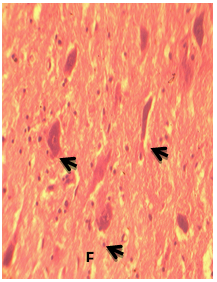
- Three DCN were identified in the cerebellum of AGR, these include; the DN (lateral, nucleus dentatus), IN (middle, nucleus interpositus) and FN (medial, nucleus fastigii). The average size of the DN cell bodies ranged from 70 - 300 µm in diameter for small to large neurones, respectively in both the dorsal and ventral regions (Table 1). The DN was the most lateral (cerebellar hemispheres) followed by the IN in between the two nuclei (paravermis) and the FN the most medial (vermis) (Fig. 1). Also, the size of the DN was larger than the other two, and had two regions; the dorsal and ventral regions. In the dorsal region, some neurones were in form of cellular band of folded bag –like shaped (Fig.2 A). Also, multipolar and bipolar neurones were observed in the two regions of DN (Fig. 2 A and 2B). The size of the IN neurons ranged from 41 – 100 µm, with an average of 50µm (Fig. 3). Both multipolar and bipolar neurones were also noted. The bipolar neurones resembled those of the FN in their staining features.The FN in the vermal region had dominantly spindle-shaped bipolar neurones (Fig. 4). Most common neurons of the FN were small and medium-sized cells, ranged between 33 – 55 µm (Table 4), with few large multipolar neurones.
|
4. Discussion
- The cerebella of AGRs examined in this work were organized into three zones; the bilateral cerebellar hemispheres, paravermis and vermis. Three pairs of DCN were observed in the white matter associated with the corresponding zones; such that, the DN was located on both sides of the lateral hemispheres, IN in the paravermis and FN in the vermis in agreement with the earlier report on the relationship between the cerebellar cortical zones and the deep nuclei in cat, rabbit and monkey [4]. Similarly, three DCN (DN, IN and FN) were reported in field vole bank, pine vole [20], chinchilla and mouse [20-21]. In human, four pairs has been documented, where, IN was subdivided into globose and emboliform nuclei [9]. Structure and topography of the cerebellar nuclei have been described in domestic animals; ruminants, the horse [22], the cat [23] and the camel. The DCN for large neurones (40-50 µm), medium-sized (25-35 µm) and small size neurons (12.5-20 µm) [24] and the shapes (multipolar or polygonal for large size neurons, polygonal or spindle shape for medium-sized and oval or spindle shaped for small neurons) has been documented [7]. The DN was the largest in their report [7] as well as in the present study. In the boar, horse and polar fox, FN was noted to be the longest of the three nuclei, but weakly developed in mole and shrew [25]. The sizes of DCN may be species specific.The present study noted various shapes of the DN, particularly the multipolar and long cellular bands in form of folded bag like shapes were more abundant but bipolar neurones were few. The cyto-architectural of DN in man and animals is said to resemble the inferior olive nucleus, because of its crumple-like appearance and a medially placed hilus [26], which may not similar to this findings. Functionally, DN is showed to concerned with voluntary movements of the extremities, including reaching and grasping [14]. The large size of the DN in man with its thin lamina, discrete borders and extensive convolutions is similar to higher primates and associated with unique features of cerebellar function such as voluntary movements of the extremities, including reaching and grasping [14]. Other authors [26] also described the function of DN as being responsible for the planning, initiation and control of voluntary movements. The present results suggest that the large DN in the AGR is related to the animal ability to use their extremities, particularly in handling and grasping of grasses and other food materials in the wild /and or captivity.The IN was observed to be larger in the AGR than grasscutter, suggesting a better function of this nucleus in the AGR than the later [27]. In the same vein, IN has is reported to be the largest nuclei in chinchilla, some rodents [28], and in the whale [16]. Base on function, the IN is considered as the principal nucleus for getting sensory information from bodily receptors; hence, whale has a large surface area to deal with [16]. It means that IN receives a large amount of sensory information from the limbs and, in turn, elaborates signals for movement control [16, 29].The paravermal cortex and the functionally associated IN are considered important structures for limb movement [6]. General, the relatively large size of IN in this study may be in part responsible for AGRs ability to use their limbs for climbing, swimming and movement in search of food and escape from enemies in the wild. This suggests a correlation between the size of the IN and its function in life animal. To further support this fact, it was documented that limb movement representations in the IN is instrumental for the control of goal-directed movements such as grasping or precise foot placement during gait [30]. This imply that the contribution of the IN may become particularly critical whenever proximal movements of the limb are coupled with precise, goal oriented, movements of the hand [31-32].The size of FN in the present study was relatively small compare to its size in the grasscutter [28], as noted in chinchilla [29]. The FN has been shown to be involved in the control of eye and head movements, equilibrium or balance, upright position and gait. Some researchers subdivided the FN into rostral and caudal components; where the rostral portion is involved in the control of somatic musculature, head orientation and eye-gaze shifts, while, the caudal portion plays key roles in generation of saccade eye movements [18]. Since FN is concerned with the control of eye movements and head orientation, grasscutter [27] may have better eyesight than AGR to support the report that ‘AGR has very poor eyesight and depends on its senses of smell and hearing’ [33].
5. Conclusions
- The DCN in the AGR were well developed and DN was the largest, followed by IN and the smallest being FN, which may be correlated with the animal high motor function such as limb movements in reaching and grasping in the course of feeding or escape from predators. The nuclei may also aid in control of eye and head movements, equilibrium or balance, to support the upright position and gait in the wild or captivity. The deep cerebellar nuclei (DCN) are the main output centers of the cerebellum, but little is known about their development, further studies on the development of DCN and their connectively in AGRs is of paramount important.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML