-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Brain and Cognitive Sciences
p-ISSN: 2163-1840 e-ISSN: 2163-1867
2014; 3(2): 44-49
doi:10.5923/j.ijbcs.20140302.03
The Neuroanatomical Localisation of a Specific Memory
Elroy P. Weledji1, Ciaran M. Regan2
1Senior Lecturer in Anatomy & Clinical Surgery, Faculty of Health Sciences, University of Buea, Cameroon
2Department of Pharmacology, University College Dublin, Ireland
Correspondence to: Elroy P. Weledji, Senior Lecturer in Anatomy & Clinical Surgery, Faculty of Health Sciences, University of Buea, Cameroon.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
We performed a study on the association between short-term memory and morphology in the striatum. The aim was to demonstrate if the memory forming processes changed the structure in the brain. An experimemental rat received the electrical shocks to be trained for the avoidance memory. Using Concanavalin a staining we found that the process of memory acquisition generated a diffuse staining on the right striatum. We suggest that memory acquisition in rats may be related to motivation and reward which have parallels in man.
Keywords: Memory trace, Developmental replay, Neural cell adhesion molecule (NCAM)
Cite this paper: Elroy P. Weledji, Ciaran M. Regan, The Neuroanatomical Localisation of a Specific Memory, International Journal of Brain and Cognitive Sciences, Vol. 3 No. 2, 2014, pp. 44-49. doi: 10.5923/j.ijbcs.20140302.03.
1. Introduction
- The essential functions of the nervous system are to receive, store and process information coming from the environment, to integrate it with previous information, and to respond to it by appropriate adaptive behavior. This system of information storage must be stable over long periods. It is highly likely that only structural changes would have sufficient stability for information storage or memory formation as proteins have a half-life of 6-14 days, RNA of up to 24hrs and lipids and carbohydrates are continually being turned over (Appel 1967). There are two dominant theories that have been proposed to govern mechanisms of memory formation. The first of these argues that increases in synaptic efficacy (as seen in long-term potentiation} may mediate processes of information storage (Anderson et al 1966, Lomo 1971, Bliss and Garden-Medwin, 1973). The other hypothesis is that changes in the pattern and connectivity of synapses may mediate these processes and this has been consistently supported by experimental evidence (Matthies 1982). Long-term memory stored in the brain last our entire lives and direct observation of images of brain cells forming temporary and permanent connections in response to various stimuli have been produced (Colicos et al 2001). They illustrate activity- dependent presynaptic structural plasticity facilitating the formation of new active presynaptic terminals. More recently, a computer model have shown how connections in the brain must change to form memories and this could help develop artificial cognitive computers (Cheu et al 2012). 2-Deoxyglucose uptake studies associated with a passive avoidance paradigm of memory acquisition in rats have indicated the striatum to be actively involved in memory formation with possible implications for the hippocampus as well, but yoked (foot-shocked) controls showed changes which were evident in the hippocampus (Kossut & Rose 1984). Immunoprecipitation studies using anti-NCAM (neural cell adhesion molecule) antibodies indicated that the striatum and hippocampus and perhaps the cerebellum were involved (Nolan et al 1987). These activity changes were quite rapid, beginning from immediately after training. Interventive methods using antibodies to neural cell-surface components were effective in inhibiting memory recall, but only when injected intra-cerebroventricularly (i.c.v) either before or 2hrs following training. This suggests the occurrence of a rapid synaptic remodeling process correlating to memory formation.
2. AIM
- This experimental study attempted to demonstrate if the memory trace of a rat, 24 hours after being trained are localised to particular areas of the striatum, by using the histochemical method of lectin (Concanavalin A) binding to the specific sugar mannose of the neural cell adhesion molecule (N-CAM).
3. Materials and Methods
- The passive avoidance paradigm of memory acquisition in rats is a procedure whereby a rat receives an electrical shock when it steps down from a platform. If it remains on the platform for 5 minutes after the shock, this indicates that the animal has acquired an avoidance memory. The yoked animal is that which is not allowed to avoid the shock.3 adult Wistar rats (250-300g), i.e. a 24 hour post-trained rat (T) and two controls, the yoked (foot-shocked) (Y), and the passive (non-shocked) (P), were killed by cervical dislocation without anaesthesia as the latter may interfere with the morphological changes in the striatum. The yoked control was used to eliminate the effect of stress on the changes in the striatum. Following dissection, the whole brains were cut at the mid-brain region and the anterior end immersed in liquid nitrogen. The olfactory lobes were cut at their bases and the length of the rest of the brain tissue was measured with a metre ruler. Each measured to about 0.5cm. Coronal sections (20um thick) were cut on a freezing microtome (-30℃), counted and numbered from the anterior to the posterior end of the brain tissue. They amounted to about 250 sections. Saggital sections had previously been shown to be ineffective in illustrating the 3- dimensional morphology of the striatum. Each section was collected on a slide, and all were kept frozen overnight at -70℃. The sections were washed three times in Dulbecco ‘A’ phosphate buffered saline (DPBS) containing 1% bovine srum albumin (BSA) and 1mm sodium azide (NaN3). Sections were incubated for 45 minutes in 20 ug/ml lectin (FITC concanavalin A) which binds mannose residues. This concentration of lectin and duration of incubation were determined from preliminary studies to be most effective in staining for the sugar residues. Control sections were incubated in 50 mM alpha methyl mannoside. This concentration of mannoside was also determined from preliminary studies to be most effective in blocking specific lectin binding.After incubation in the lectin, the tissue sections were washed with DPBS containing 1% BSA and 1mM NaN3 and then post-fixed for 10 minutes in 40% glutaraldehyde in DPBS buffer. Preliminary studies had indicated a better resolution of stained sections that were post fixed as compared to pre-fixed or non-fixed sections. These were then washed in DPBS buffer and mounted on gelatin-coated slides with 20-80% DPBS/glycerol for examination of both the left and right cortex and striatum by fluorescence microscopy. The sections examined were related to the Bregma Scale of the Atlas of the rat brain in stereotaxic coordinates (Paxinos & Watson. 1982) Corresponding section numbers in the control animals, the yoked and passive were compared. The sections examined critically were section numbers 105-115 (relating to Bregma 6.7-2.2mm) for the frontal cortex and section numbers 180-230 (relating to Bregma 1.7-1.3mm) for the striatum, as preliminary studies had indicated increase in lectin binding in these regions.
4. Results
- I. Section 105 relating to Bregma scale 3.7mm indicated a homogenous staining of the right and left frontal cortex but with a right staining of discrete cell bodies.II. Sections 217 and 219 corresponding to Bregma 0.2-0.3mm and to 4340-4380um in from the olfactory bulb stem, indicated diffuse staining just below the cortical structure, in the ventrolateral surface of the striatum. The cells of the striatal patches on the right being very much brighter than on the left.III. In the animal’s right striatum with reference to the Bregma area 0.2-0.3mm, in the ventrolateral region, an increase of concanavalin A binding was apparent.IV. Both controls, the passive and the yoked did not show this. Mannoside removed all staining, thereby indicating that the staining was specific for mannose.V. The lining of both ventricles stained brightly and equally.VI. The cortex on both sides stained poorly but equally.VII. Therefore, it appears that there is a localized but diffuse staining associated with memory acquisition of a passive avoidance paradigm.
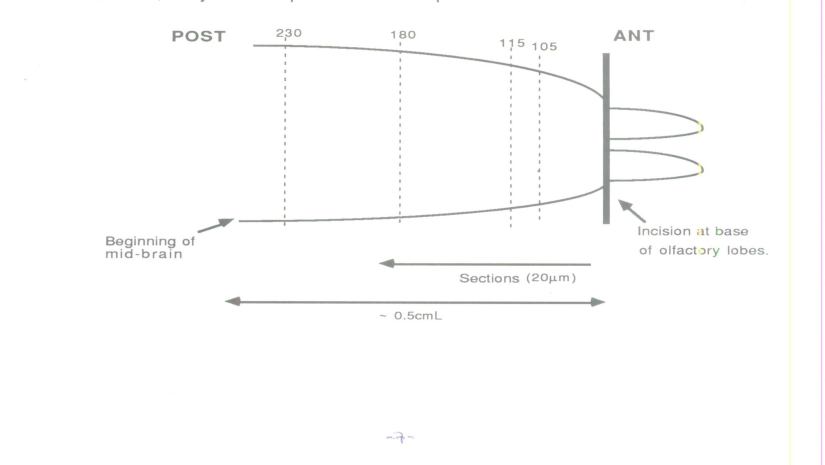 | Figure 1. Schematic representation of dissection |
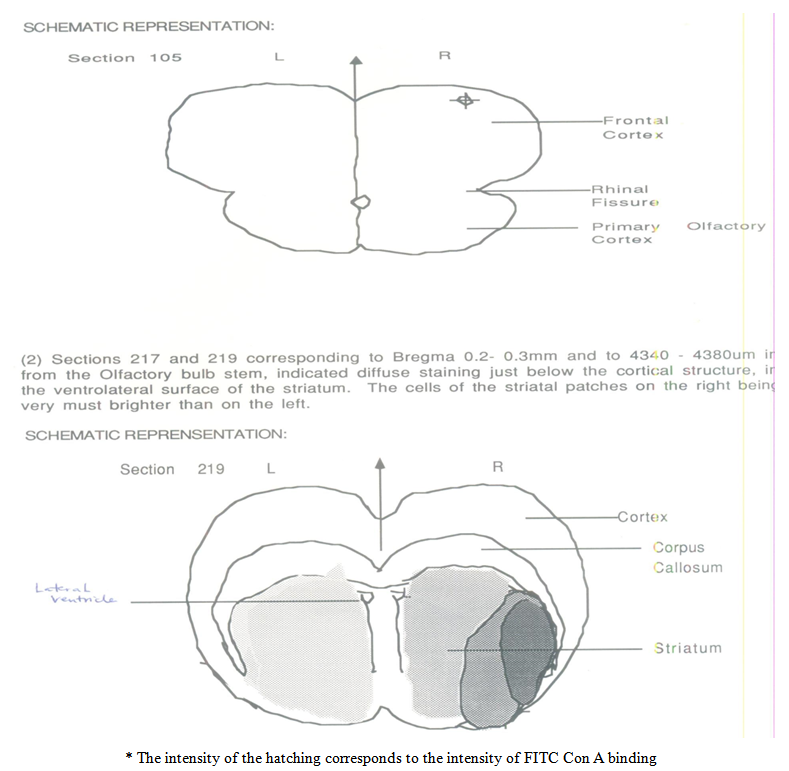 | Figure 2. Schematic representations of Section 105 and 219 |
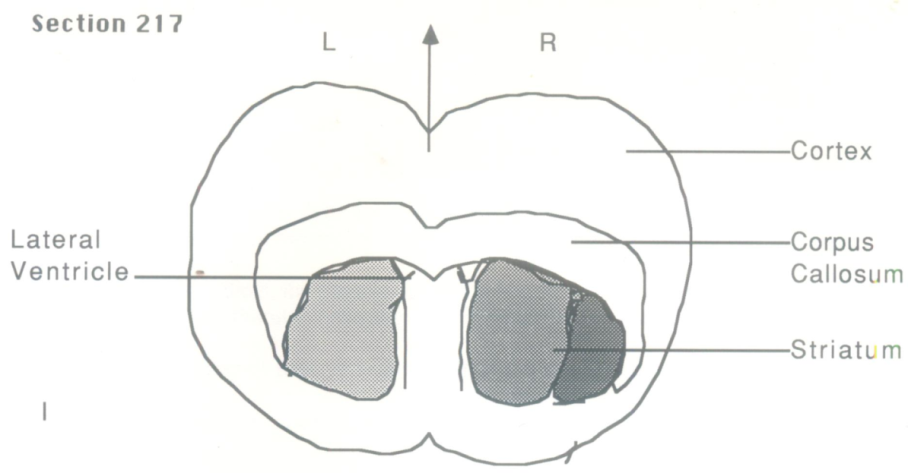 | Figure 3. Schematic representation: Section 217 |
5. Discussion
- In this study 20um thick coronal sections of the frontal cortex and the striatum of a 24-hours post-trained adult rat, and two controls, (1) a passive adult rat, and (2) a yoked adult rat, were stained histochemically with plant lectin, (concanavalin A) specific for mannose sugar. On fluoroscopic examination, there was an asymmetric and discrete staining of the right frontal cortex of the trained animal related in stereotaxic coordinates to 3.7mm on the Bregma scale of the 'Atlas of the rat brain' and a diffuse staining of the ventrolateral area of the right striatum relating to 0.2-0.3mm on the Bregma scale. Both controls (the passive and the yoked) did not show this and mannoside removed all staining. It appears therefore that a diffuse staining of the striatum is associated with memory acquisition and may suggest that memory acquisition is related to motivation and reward.The diffuse staining of the striatum suggests that the morphological changes associated with memory acquisition may not be discrete and localized. However, the reverse may occur in the frontal cortex where there is an observed discrete staining of cell bodies. It is interesting to note that the diffuse staining of the ventrolateral area of the striatum corresponds to the area encompassing the dopaminergic (DA) inputs from the substantial nigra (nigrostriatal) of the mid-brain and the mesocorticolimbic pathways. The nigrostriatal system is related to motor functions as evidenced by the movement disorderes which accompany the degeneration in Parkinson’s disease (Hornykiewics 1966). The mesocorticolimbic areas are thought to be related to mood (Heimer & Wilson 1975). The staining in this area may indicate that memory acquisition is related to motivation and reward (Fibiger and Phillips 1986). The fact that Parkinsonian patients do suffer from amnesia may also indicate an intricate and complex link between the nigro-striatal pathway and memory acquisition. The striatal patch neurons may also project to the location of dopaminergic (DA) cell bodies and these neurons may also receive inputs from the striatal matrix neurons that project to the non-dopaminergic system of the substantia nigra and thr mesolimbic system (Gerfern 1976). The effect of DA antagonists e.g. the anti-schizophrenic drug, trifluoperazine has been shown to exert an effect at both a very early and late stage of memory formation, as determined by memory deficits incurred in passive avoidance learning (Niemegeers et al 1969). Recently, the ‘atypical’ neuroleptics have been shown to be effective as anti-psychotic drugs without producing dyskinesias (Inchihara et al 1988, Levin et al 1986). Thus, these systems may be heterogenous, as also evidenced by the report of the existence of two sets of physiologically distinct nigro-striatal neurons, and the pharmcological/physiological differences between mesocortical neurons that are dependent on their cortical targets (Shepard and German 1987, Chiodo et al 1984). In Ahlzeimer’s disease, where plaque deposits affect the neurofibrillar protein network, a replay of N-CAM neurodevelopmental events in memory formation may be inhibited, and in ageing, the loss of neurons may limit the synaptic connectivity changes associated with memory acquisition and consolidation. The fact that the yoked control animal did not show these histochemical changes is an indication that the effect of stress on the morphological changes in the striatum during the acquisition of the memory task in the trained animal was minimal. More likely, the effect is manifested in the hippocampus and other limbic areas, as shown by the increase in 2-deoxyglucose uptake predominantly in the hippocampus of a yoked animal. The asymmetric staining of discrete cell bodies in the right frontal cortex may relate to the asymmetric staining of the right striatum. The asymmetry of staining may be due to a predominance of activity of one side of the brain over the other with regard to the parameter being studied. The projection from the prefrontal cortex to the striatum, and the functional correlates of this connection is well supported by anatomical and behavioural (cognitive) studies respectively (Oberg & Divac 1976). However, some ‘higher cortical functions’ appear to be independent of this efferent link, e.g. language seems to be resistant to basal ganglia disease. It is possible that the most recently involved functions of the human brain do not involve the neo-striatum, but follow other routes to efferent mechanisms. Memory ‘patterns’ are known to exist in non-thalamically dependent temporal neocortex (Bowsher 1981), and so it would be interesting to study this area in the rat histochemically. It would also be valuable to study the cerebellar cortex with this histochemical procedure, as it is endowed with a more organized, layered and heterogenous cellular distribution unlike the homogenous distribution of the striatum. Also encouraged is the fact that the cerebellum is directly or indirectly involved in learning of skilled movements with retention of proprioceptive information, and it serves as a special guide for handling of rather diffusely organized proprioceptive information. Moreover, it receives inputs from the cortex through the striatum.One of the short-comings of this study is that the sample size is too small as only one rat was used in each group. An increase of sample size to 4-6 rats in each group would have made the results more tenable. There were some weaknesses in the experimental method. As N-CAM is present in all neurons and Man NAc incorporated into all glycoproteins, direct immunofluorescence studies using anti-NCAM, or autoradiographic studies using 3H-N-acetyl-D-mannosamine (Man NAc), a sialic acid precursor, may not have sufficient resolution to demonstrate local change. However, the use of an antibody specific for the alpha-2,8 sialic acid linkages of resialylated N-CAM may prove useful. The expression of PSA-NCAM which contributes more specifically to the structural plasticity in the brain would be better tested. This is especially so as the anti- PSA –NCAM antibody works well in the staining of rat brains. (Farima Bouzwouk et al 2001) As the results on the striatum were not easily reproducible in many of the sections, perhaps it may be better to look at the striatum of a 12 hour post-trained rat in which time the memory acquisition process may be more intense. It would be suggested that real pictures of the slides, video imaging on the right striatum (particularly the ventrolateral aspect) or higher resolution with electron microscopy may show the morphological and functional changes occurring during memory acquisition.
ACKNOWLEDGEMENTS
- This experimental study on animals was given ethical clearance by the ethical committee of the Health Research Board of Ireland. The study was an academic thesis originally presented to the Health Research Board of Ireland in October 1989 but never published. The project was supported by a grant from the Health Research Board of Ireland. My gratitude also goes to the Postgraduate department of Medicine and Surgery at University College Dublin for giving me access to their freezing microtome. Special thanks to Mrs Emer Leahy, (postgraduate student) for helping me with her special skills in training the animals, and her constant advice on the procedure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML