-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2024; 14(3): 51-58
doi:10.5923/j.ijaf.20241403.01
Received: Nov. 13, 2024; Accepted: Dec. 10, 2024; Published: Dec. 21, 2024

Post-Disturbance Recovery and Species Composition in the Western Mau Forests Complex, Kenya: Insights into Floristic Dynamics and Ecological Resilience
Festus M. Mutiso1, Patrick C. Kariuki2
1School of Agriculture, Environment, Water & Natural Resources, South Eastern Kenya University (SEKU), Kitui, Kenya
2Geothermal Energy Training and Research Institute (GeTRI), Dedan Kimathi University of Technology (DeKUT), Nyeri, Kenya
Correspondence to: Festus M. Mutiso, School of Agriculture, Environment, Water & Natural Resources, South Eastern Kenya University (SEKU), Kitui, Kenya.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The Mau forests complex is one of the few remaining indigenous forests in East Africa. However, anthropogenic activities have reduced the forest cover, biodiversity and compromised its many ecological, economic and socio-cultural roles. This paper examines the post-disturbance recovery process in a section of Western Mau forest. A total of 26 species was recorded. Dombeya goetzenii had the highest frequency, density, relative density, and species importance value. Prunus africana had the highest basal area and relative dominance. Tree was thedominant life form. Out of the 26 species, 7 species fall under the category of pioneer species while 19 fall under the climax species. Majority of the species (20) originated from Afromontane/Guineo-Congolian while 5 and 1 were from Afromantane and Guineo-Congolian respectively. Euphorbiaceae was the most species-rich family followed by araliaceae then oleaceae and rosaceae families. The study concluded that most of the anthropogenic disturbances have ceased and the ecosystem is on its post-disturbance recovery pathway. Dominance by species of Afromontane/Guineo-Congolian origin is an indication that the site maintained lose its ecological resilience after disturbances. Continuous efforts to keep off disturbances and monitoring of regeneration patterns and successional pathways are recommended.
Keywords: Tropical forests, Mau ecosystem, Western Mau forest, Floristic composition, Species richness
Cite this paper: Festus M. Mutiso, Patrick C. Kariuki, Post-Disturbance Recovery and Species Composition in the Western Mau Forests Complex, Kenya: Insights into Floristic Dynamics and Ecological Resilience, International Journal of Agriculture and Forestry, Vol. 14 No. 3, 2024, pp. 51-58. doi: 10.5923/j.ijaf.20241403.01.
Article Outline
1. Introduction
- Mau forests complex is one of the few remaining indigenous forests in East Africa [1]. According to [2], the forest cover of Mau forests complex has reduced overtime. The ecosystem is a biodiversity sanctuary that lies across the Equator between 0° 1’ 0” N and 0° 55’ 0” S latitudes and between the longitudes of 35° 15’ 0’’ and 36° 15’ 0” E [3]. It holds high species diversity as indicated by [4] who recorded 50 species and [1] who recorded 61 species of trees of which four genera are endemic. Past and present anthropogenic disturbances have continued to interfere with the ecosystem recovery process, species composition, diversity and richness. According to [5], in the year 2005, satellite imageries showed that 8,214Ha of forest cover had been lost within the Mau forests complex. Past selective logging targeting high value climax species such Prunus africana has greatly affected the forest complex. According to [6], the abundance and population structure of P. africana in Western Mau forest had varied spatial distribution due anthropogenic activities such as de-barking, logging, and animal grazing. In a different study in Mau ecosystem, [7] cited alteration of cyclic and parallel configuration processes caused by anthropogenic disturbances and thereby affecting future ecosystem resilience. Additionally, [8] noted low species diversity in Mau forests complex which was largely attributed to selective logging targeting species such as P. africana, Podocarpus latifolia, and Olea capensis. Elsewhere, [9] attributed lower species diversity in a disturbed site is attributed to anthropogenic disturbances. These previous studies indicate an ecosystem that was heavily disturbed by anthropogenic activities resulting to changes in floristic composition. As such, this underscores the importance for further studies to document the post-disturbance recovery process with a focus on floristic composition and species richness.Post disturbance studies have highlighted different species composition and successional pathways in different sections of the Mau forests complex. For instance, [10] described the Mau ecosystem as a sea of Neoboutonia macrocalyx with isolated stands of Juniperus procera several years after disturbances. In a different study in Mt. Blackett and Kedowa Blocks of Mau forests complex, [7] reported individualistic successional pathway in Mau characterized by invasive species such as Trichlodus ellipticus. [4] reported that forest clearing had reduced forest basal area and forest stocking of big trees. Further, species importance values showed a reduction in commercially valuable species while colonizer and fire tolerant species were predominant. The effects of disturbances have also had effects on the carbon sequestration ability of the Mau forests complex. For instance, [11], reported the effects of disturbances on carbon stocks both in biomass and soil stock in Mau forests complex and as such they recommended that for effective management of forest towards climate stabilization, then disturbance should be minimized if not avoided. Though the Mau forests complex has been subjected to many anthropogenic disturbances, studies have shown an ecosystem that is progressively moving towards recovery. For instance, [12] documented that though the Mau ecosystem showed signs of recovery after evictions of the “Ndorobos”, it will take a long time to regain its pre-disturbance stand structure. In a study in Mau forests complex, [13] reported secondary regrowth which was an indicator that the forest was resilient to perturbations and had the potential to recover. In Kedowa Block of Mau forests complex, [7] recorded stable successional stages which conferred the ecosystem the much needed resilience necessary for post–disturbance restoration of ecological integrity. The Kedowa Block had a well balanced trajectory successional pathway as evidenced by a balance in the occurrence of the colonizers, followers and climax species. A different study by [14] reported greater abundance and density of shade-dependent species on all studied sites indicating that pioneer species were giving way to shade tolerant species is a natural process. Presently, Mau forest canopy is dominated by colonizers and is slowly graduating into climax forest if disturbances are controlled [1]. These previous studies have given insights on the post-disturbance recovery process, resilience of the ecosystem as well as post-disturbance successional trajectories. Additional studies are, therefore, important in monitoring the post-disturbance recovery process and plant successional pathways. Such studies can give insights on whether the recovery process in on the right trajectory and whether additional interventions are required to restore the tropical ecosystem to its pre-disturbance status.
2. Material and Methods
2.1. Description of the Study Sites
2.1.1. Geographical Location and Forest Status
- The study area lies between 0.702392° and 0.720048° South latitudes and 35.641658° and 35.649672° East longitudes. The physiography of the study site is mainly characterized by highlands covered by indigenous vegetation. The sampling section covered 2 Kilometer stretch with a total acreage of 12 acres. The Mau forests complex forest cover has been changing overtime due to anthropogenic disturbances. Figure 1 shows the mapped forest in year 2004 while figure 2 shows the current status of the forest that shows a substantial encroachment over the period of approximately 20 years.
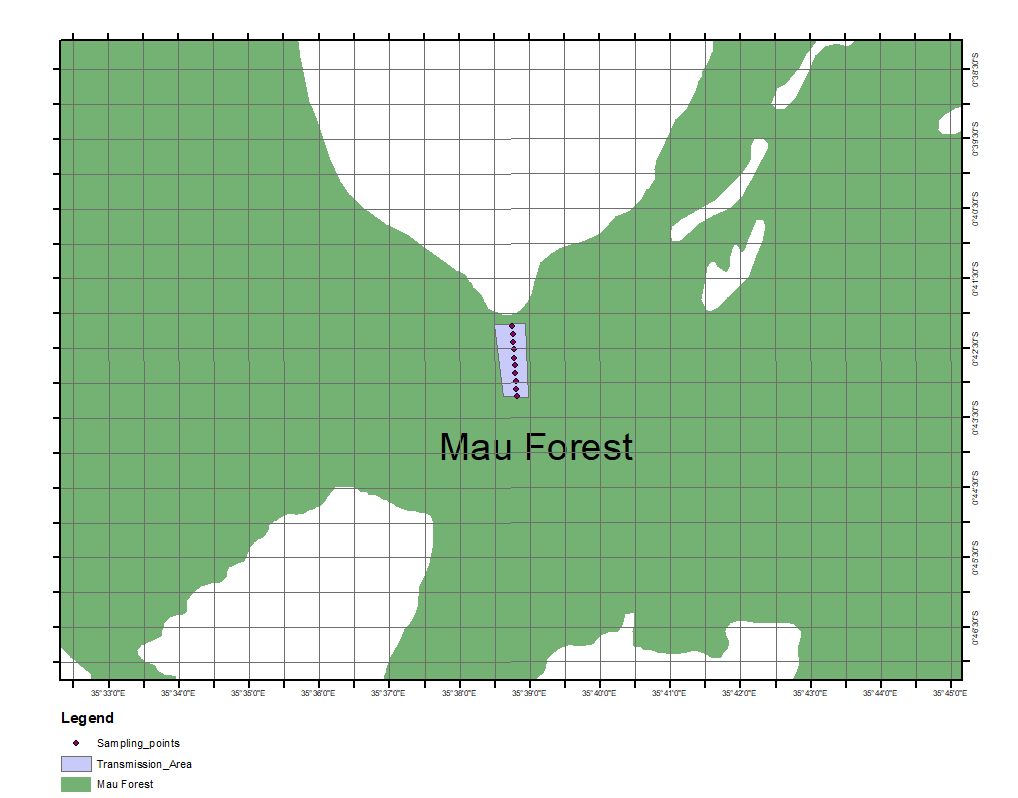 | Figure 1. Status of Mau Forest Complex as of 2004 Landcover Mapping (Source RCMRD, 2004) |
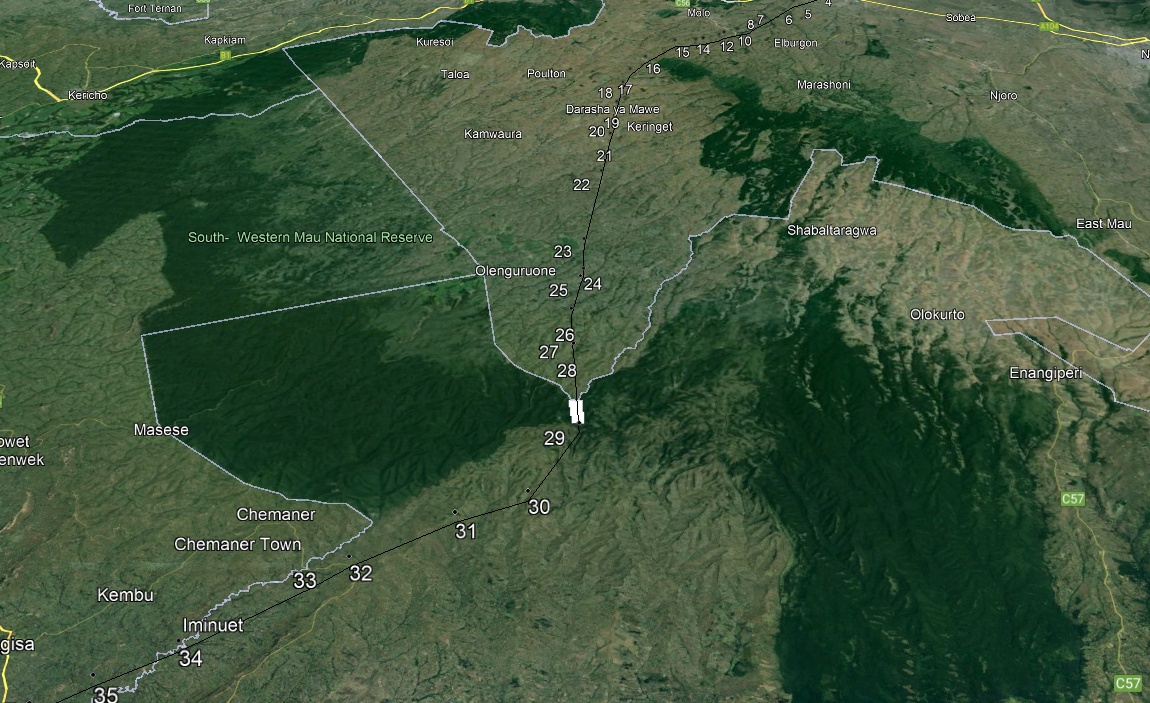 | Figure 2. Google Earth Image of the current status of the forest showing the sampling section |
2.1.2. Hydrogeology / Hydrology
- The hydrogeology of the area is controlled by the geology, structural characteristics, and rainfall. The study site and its environs are drained by Amala river. The site forms a major catchment of the river regime that serves a wide catchment with the hydrogeological characteristics controlled by the geology whose landscape comprises of a volcanic highland.
2.1.3. Geology and Soils
- According to [15], the study site is characterized by tertiary to recent volcanic rocks. These are volcanic rocks that have originated from the volcanic centres (including Mau, Tinderet, and Londiani) and fissures associated with the Rift Valley. The rocks include phonolites and trachytic lavas and volcanic pyroclastics (tuffs and ashes). The lava is hard and compact rocks formed by high temperature magma. The tuffs are consolidated ashes with different welding degrees. The ashes are unconsolidated volcanic materials ejected from violent volcanic activities and are mainly along the floor or the rift. The soils are mainly loamy in the study site with clay soils in the lowland sections of the transect.
2.2. Sampling Design and Data Collection Techniques
- A 2km transect was run across a section of Western Mau Forests Block (Figure 1 & 2) and 10 contagiously distributed sampling plots established. Each plot, which was circular in shape, had a radius of 11.5m. In each plot, all trees (dbh ≥ 5 cm) were identified by their scientific/local names and diameter at breast height (DBH) measured.
2.3. Methods of Data Analysis
- The field data was summarised and organised in tabular form ready for further processing. The species Frequency (F), Density (D), Relative Dominance (RDo), Basal Area (BA) and Species Importance Value (SIV) were calculated. RDo was obtained by dividing the dominance by the sum of the dominance of all species, multiplied by 100. SIV = RA+RD+RF while BA = DBH^2 x 0.005454. The families, scientific names, life forms, succession type, stage mainly occurring, preferred habitat and geographical origin were identified to capture floristic composition and species richness.
3. Results
3.1. Species Occurrence, Stocking, Basal Area and Importance Value
- A total of 26 species were recorded in the sampled section. Dombeya goetzenii had the highest frequency (8), density (156.53 stems/Ha), relative density (34.95%), and species importance value of 63.76 as shown in Table 1 below. Prunus africana had the highest basal area (2.31m2/ha) and relative dominance (19.57%). Cussonia holstii, Cassipourea malosana, Croton megalocarpus, Scutia myrtina, Croton macrostachyus, Diospyros abyssinica, Rothmannia urcelliformis, Acacia abyssinica and Celtis africana had the lowest frequency and stocking levels. Diospyros abyssinica had the lowest basal area, relative dominance (0.02%) and species importance value of 1.99 (Table 1). Two top densest species; Dombeya goetzenii and Dracaena afromontana contributed 54.3% of the total stocking of the sampled area. In some instances of the sampled areas, Dombeya goetzenii formed mono-dominant stands (Figure 3).
|
 | Figure 3. A mono-dominant stand of Dombeya goetzenii in a section of the sampled area |
3.2. Species Floristic Composition
- Tree was the dominant life form (representing 23 species) while shrub and shrub/tree life forms had the least of 2 and 1 species respectively. Out of the 26 species, 7 species fall under the category of pioneer species while 19 fall under the climax species in early and late successional stages (Table 2).
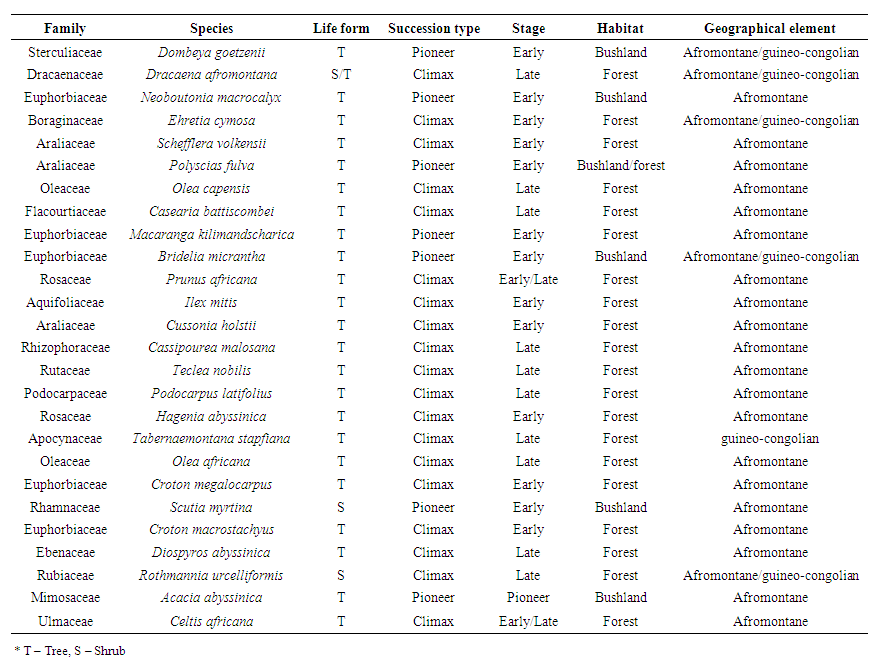 | Table 2. Floristic composition, succession, habitat and geographical element of identified flora |
3.3. Species Richness
- Euphorbiaceae was the most species-rich family with 5 species followed by araliaceae with 3 while oleaceae and rosaceae families had 2 species each (Table 3).
|
4. Discussion
4.1. Species Occurrence, Stocking, Basal Area and Importance Value
- The recorded number of species was generally low for a tropical forest ecosystem. The low species diversity is an indication of a forest ecosystem recovering from anthropogenic disturbances. In a different study in Kerisoi forest of Mau forests complex, [8] recorded similar low species diversity in which was attributed to anthropogenic disturbances mainly selective logging. While this study recorded 26 species, Joyce’s study recorded 20 species; a clear indication of differences in levels of disturbances in different sections of Mau forests complex. The observed low species diversity may also be attributed to closeness of some of the sampling points to forest edges. According to [16], such closeness may cause edge effect that impact species diversity. Similar edge effects on species diversity have been reported by [17] and [18]. According to [19], such low species frequencies and relative frequencies indicate a poor distribution of species.The species importance value of Dombeya goetzenii indicated that it was the most dominant species in the study site.Such dominance can be attributed to past anthropogenic disturbances since D. goetzenii is a post-disturbance pioneer species that characterizes tropical ecosystems at the early seral stages of succession. This also explains why in some sections of the study site the species formed mono-dominant stands. However, its presence in the study site should not be a major concern since ecologically, it’s supposed to be replaced by the many climax species at the site in the final stages of succession. This is further supported by [14] who found out that pioneer species give way to shade tolerant species in a natural process, thus making them the worst variants in gap regeneration. The observed overall stocking is generally good for a recovering tropical forest. However, a stocking where two species namely Dombeya goetzenii and Dracaena afromontana constitutes over 50% is an indicator of a poorly balanced forest ecosystem in terms of species stocking. In a different study in Mau forest ecosystem, [4] attributed reduced stocking to anthropogenic disturbances. It is likely that past anthropogenic disturbances did not wipe out all the high value tree species such as Prunus africana as evidenced by its relatively high basal area in this study. It is important to note that Prunus africana is listed as vulnerable (VU) under the International Union for Nature Conservation (IUCN) red list and as such should be accorded high conservation status compared to other species captured in this study. Further, P. africana was listed under CITES in East Africa in 1995 though its exploitation still occurs in member states who are signatories to the Convection. Further, [6] largely attributed exploitation of P. africana to human activities such as debarking and selective logging. In a different study in Kakamega tropical rain forest, [20] and [21] documented loss of P. africana caused by human debarking where the barks were used for medicinal purposes in the pharmaceutical industry.
4.2. Floristic Composition
- The high number of climax species in their early and late successional stages is a clear indicator that the forest ecosystem is on its way to recovery. Though the pioneer species occupy slightly over half of the stocking level, their stocking is likely to drop progressively as the forest passes through the various seral stages. Our findings are further supported by [1] who stated that Mau forest canopy is currently dominated by pioneer species and is slowly moving towards climax stage provided that the disturbances are kept under control. In a different study in Mt. Blakett and Kedowa blocks of Mau forests complex, [7] recorded a similar high percentage of colonizer species. It is important to note that most of the anthropogenic disturbances such as selective tree logging and human encroachment in Mau forest stopped less than 15 years ago. For instance, analysis of satellite imageries of Western Mau forest between 1973-2005 showed that a total of 8214 Ha of the forest had been lost to human encroachment [5]. The high concentration of species of Afromontane/Guineo-Congolian origin is largely attributed to the location of the study site in respect to the equator. The Afromontane/Guineo-Congolian zone strands along the equator and shows a high concentration of species endemism thus underscoring the conservation value of the forest ecosystem under study. According to [3], Mau forests complex is a biodiversity sanctuary and is the largest indigenous forest in East Africa that lies across the Equator between 00 1’ 0” N and 00 55’ 0” S and between the latitudes of 350 15’ 0’’ and 360 15’ 0” E.
4.3. Species Richness
- The fact that only 4 families had more than one species is an indicator of a forest with low species richness. [8] in a different study in Kerisoi forest of Mau forests complex recorded even a lower species richness where only three families had more than one species. The high species richness observed for euphorbiaceae family is likely to have been caused by past anthropogenic disturbances since three out of the five species are pioneer species. Such pioneer species tend to occupy disturbed forest ecosystems at the early stages of succession. Our findings are in consistent with [5] who in a different study in Mau forest complex noted the presence of disturbance indicator species like Neoboutonia macrocalyx, Croton megalocarpus and Vernonia auriculifera in the vegetation community which was attributed to considerable levels of human disturbance in the forest and representing retrogression in the vegetation succession pathway. Additionally, [10] described the Mau ecosystem as a sea of Neoboutonia macrocalyx with isolated stands of Juniperus procera several years after disturbances. It is likely that members of oleaceae family (O. capensis and O. africana) and rosaceae family (P. africana and H. abyssinica), which are climax and high value tree species highly targeted by illegal loggers, withstood past anthropogenic disturbances. The presence of such key species is an indicator of a forest ecosystem with resilience against disturbance. Such resilience is critical in post-disturbance recovery as well as in the stability of the forest ecosystem. Similarly, [22] and [7] reported the importance of the ability of a tropical forest ecosystem to absorb disturbances without undergoing fundamental changes.
5. Conclusions and Recommendations
5.1. Conclusions
- The study has concluded that most of the anthropogenic disturbances at the study site have ceased. The site is in its early stages of post-disturbance recovery as evidenced by low species diversity and species stocking levels, species dominance and species importance value dominated by pioneer species such as Dombeya goetzenii and Dracaena afromontana. Despite the high anthropogenic disturbances, some climax species withstood disturbances as evidenced by relatively high basal area of Prunus africana.In terms of species composition, the study can authoritatively conclude that the tree life form was dominant. Though the pioneers form the bulk of the stocking levels, the dominance of the tree life form is an indicator of a forest ecosystem on the recovery pathway. Dominance by species of Afromontane/Guineo-Congolian origin, which is typical of forest ecosystems within the vicinity of equator, is a clear indication that the study site did not lose its ecological resilience following many years of anthropogenic disturbances.Lastly, the study concluded that anthropogenic disturbances targeted species of high economic value as evidenced by low species richness of oleaceae and rosaceae families and high species richness of euphorbiaceae family. While oleaceae and rosaceae families are composed of climax species, euphorbiaceae family is composed of pioneer species.
5.2. Recommendations
- We strongly recommend continuous efforts to keep off anthropogenic disturbances from the study site. Fencing off of areas where the local people were evicted from the forest will go a long way in avoiding anthropogenic disturbances.In areas of the study site where local people were recently evicted, there is need to monitor the regeneration process and successional pathways and take remedial actions where natural regeneration is not taking place or where individualistic successional pathways are evident.
ACKNOWLEDGEMENTS
- The authors acknowledge the inputs of the field crew. Review of the paper by professionals from KEFRI, South Eastern Kenya University and Dedan Kimathi University of Technology (DeKUT) is highly appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML