-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2024; 14(2): 25-39
doi:10.5923/j.ijaf.20241402.01
Received: Jun. 16, 2024; Accepted: Jul. 3, 2024; Published: Jul. 6, 2024

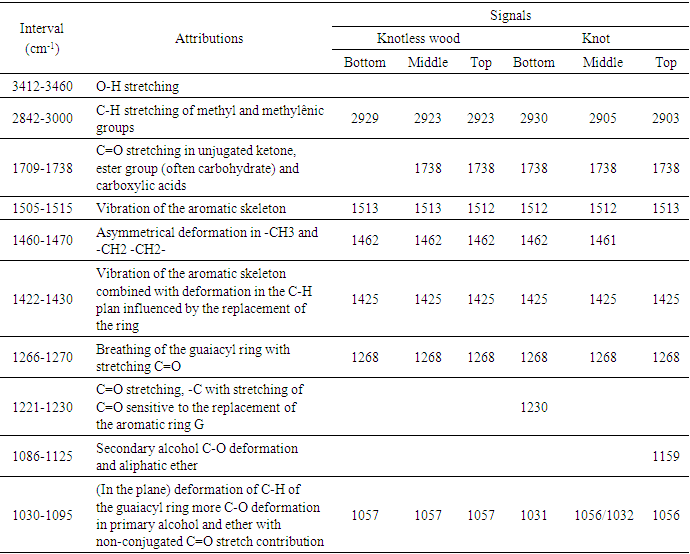
Presence of Lignin and Cellulose Intensity Signals in Knotted and Knotless Wood of Pinus elliottii var. elliottii Engelm
Deise Amaral de Deus1, André Scarambone Zaú2, Graciela Ines Bolzon de Muniz3, Silvana Nisgoski3, Heber dos Santos Abreu4, Dráuzio Correia Gama5
1Institute of Agricultural Sciences, Federal Rural University of the Amazon, Belém-PA, Brazil
2Department of Environmental Sciences, Federal University of the State of Rio de Janeiro-RJ, Brazil
3Department of Forestry Engineering and Technology, Federal University of Paraná, Curitiba-PR, Brazil
4Madeira Chemistry Laboratory, Federal Rural University of Rio de Janeiro, Rio de Janeiro-RJ, Brazil
5Center for Agricultural, Environmental and Biological Sciences, Federal University of Recôncavo da Bahia, Cruz das Almas-BA, Brazil
Correspondence to: Dráuzio Correia Gama, Center for Agricultural, Environmental and Biological Sciences, Federal University of Recôncavo da Bahia, Cruz das Almas-BA, Brazil.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The fundamental chemical components of wood (cellulose, hemicellulose and lignin) are anatomically inherent and vary along the trunks of each individual tree. The aim of this study was to analyse the formation and concentration gradient of lignin and cellulose intensity signals between knotless wood, the knot-to-wood transition zone and knots in the species Pinus elliottii var. elliottii Engelm. Wood samples in knotted and knotless regions of Pinus elliottii var. elliotti trees from a plant shop in Agudos-SP, were analysed in the laboratory of the Federal University of Rio de Janeiro. Based on the degradative methods of Klason Lignin, 13C spectroscopy and nuclear magnetic resonance and Fourier transforminfrared and histochemical tests in histological sections, differentiated patterns in the development dynamics between the anatomical elements of knotless wood and knotted wood were verified. The structure of lignin in knotted and knotless wood was different, despite the proximity of these regions to one another. Crystalline cellulose signals were more intense in the middle knot and top knot regions. It is possible to conjecture that these characteristics interfere with wood resistance in these regions along the trunk.
Keywords: Trunk Regions, Anatomical Elements, Spectroscopic Methods, Resistance
Cite this paper: Deise Amaral de Deus, André Scarambone Zaú, Graciela Ines Bolzon de Muniz, Silvana Nisgoski, Heber dos Santos Abreu, Dráuzio Correia Gama, Presence of Lignin and Cellulose Intensity Signals in Knotted and Knotless Wood of Pinus elliottii var. elliottii Engelm, International Journal of Agriculture and Forestry, Vol. 14 No. 2, 2024, pp. 25-39. doi: 10.5923/j.ijaf.20241402.01.
1. Introduction
- Cellulose, hemicellulose, lignin, and extractives are considered fundamental chemical components of wood, and can vary as a result of silvicultural treatments, the anatomical characteristics inherent to each individual and the position along the bole of a tree (Oliveira, 1997; Silva, 2002; Gatto et al., 2008; Deus et al., 2022). However, the quantities of these components do not differ significantly between species (Oliveira, 1997; Silva, 2002).The chemical heterogeneity of wood, while increasing its potential uses, represents an inconvenience to the transformation and processing industry, since it pejoratively interfereswith the technological properties required for the use of wood [Oliveira, 1997; Silva, 2002; Gatto et al., 2008). For example, variation in lignin influences hygroscopicity, the behaviour of dimensional variations and consequently, wood quality. While hemicellulose has a greater water absorption capacity and increases dimensional variation, lignin, which is essentially hydrophobic, promotes the opposite action. The higher the lignin content in the wood, the greater its resistance to atmospheric water absorption and the lower its dimensional variation (Oliveira, 1997; Silva, 2002; Gatto et al., 2008; Deus et al., 2022).Furthermore, many physical and mechanical properties of wood depend on the presence of lignin (Britez and Nogueira, 2996; Deus et al., 2022). For example, basic density is one of the most studied properties and is used as a factor for assessing wood quality, directly influencing its use. The greater the lignin deposition in the cell wall, the better the density value, a characteristic which is required for the structural use of wood (Britez and Nogueira, 2996).Understanding the mechanism of lignin formation still requires further theoretical and experimental research, especially in terms of relating lignification to the quality of the final product in the various areas of the forestry sector. Content control and the modulation of lignin biosynthesis, for example, offers a technological advance for the partial removal of lignin from woody tissues, a process which requires high investment, especially due to its importance in the cellulose pulp industry (Deus et al., 2022 (Abreu et al., 2004; Deus et al., 2023).The reduction in lignin content, which has already been associated with a reduction in the resistance capacity of trees, can be studied from other perspectives. Studies have shown that young branches are able to develop compensatory mechanisms for maintaining the structural, biophysical and biomechanical properties of tissues, despite a 64% reduction in lignin content ((Patten et al., 2007; Deus et al., 2022).However, it is worth noting that the major component of wood is cellulose, comprising approximately 50% of wood, in both conifers and hardwoods (Fengel and Wegener, 2003), with chemical and physical properties and a supramolecular structure fulfilling its function as the main component of cell walls (Klock et al., 2005).The structural organisation of cellulose is found both in both its amorphous and crystalline form. Crystalline cellulose corresponds to two thirds of the cellulose present in wood and is characterised as being more robust and resistant to heat, while the amorphous form is more sensitive, and assumes a similar characteristic to hemicellulose, in this regard, and is responsible for the absorption of water molecules, due to the empty spaces in its structure (Wilkie, 1961; Nishiyama et al., 2002; Oliveira, 2009).Furthermore, there are no well-defined boundaries between the amorphous and crystalline regions, but there is a transition from the arrangement of cellulose chains to the ordered or amorphous state (Mokfienski, 2004). Even a combination of heat + water, allows for the conversion of the amorphous to the crystalline region, where an increase in the crystalline region is associated with the reorganisation of amorphous cellulose combined with its partial degradation (Stam, 1964; Fengel and Wegener, 2003).The more crystalline the structure of cellulose, the more limited the access of compounds to functional groups and chemical bonds (Thygesen et al., 2005). The crystallinity of cellulose is an important factor impacting wood properties, changing the direction of production and even defining the quality of the final product (Dufresne and Belgacem, 2013).Like lignin, cellulose is closely related to the technological properties of wood products. The tensile strength of fibres and the elasticity modulus of wood parts, depend on and vary according to the cellulose content present in cells (Klinke et al., 2000; Lahr et al., 2018; Marvila et al., 2021).The hypothesis of the influence of lignin on the formation of cracks in wood (Abreu et al., 2004), demonstrates the need for further studies on cell wall lignification. As knots and their surroundings are largely affected by cracks, the study of lignin in these regions in justified by the need to increase wood quality. Thus, the aim of this study is to analyse the formation and concentration gradient of lignin and cellulose intensity signals between knotless wood, the knot-to-wood transition zone and knots in the species Pinus elliottii var. elliottii Engelm.
2. Materials and Methods
- Five trees of the species Pinus elliottii var. elliottii Engelm from a plant shop located in the municipality of Agudos, in the State of São Paulo, at the coordinates Lat: 22°53'20" S and Long: 47°04’39” W, were studied. The wood of the species was identified, felled and deposited in the Xylotheque of the Rio de Janeiro Botanical Gardens, with the records 10191, 10192, 10193, 10194 and 10195. Three disks were extracted from each tree, containing at least one knot/branch from the bottom (25% of bole height), middle (50% of bole height) and top (100% of commercial height) positions.Samples of knotted and knotless regions were taken from the disks, from which small wood chips were obtained using a machete. The samples were taken to the Chemistry Laboratory of the Institute of Exact Sciences at the Federal Rural University of Rio de Janeiro (UFRRJ).Using a Willey 340 (TE 040 model) knife mill, the chips were converted into sawdust for subsequent granulometry reduction in a rotating ball mill to obtain the material for the analyses.For the material extraction process, a Soxhlet device was used, using 25 grams of ground wood and homogenous wood separately, from the parts of the disk with and without knots. The material was packaged in a cartridge made with filter paper and placed inside the extraction tube. The solvents were placed in a 500mL flask, following the eluotropic scale in increasing order of polarity (cyclohexane; ethyl acetate; methanol). The extraction time for each solvent was 24 uninterrupted hours (Browning, 1967). Following this period, the extract was concentrated in a rotavapor, and the concentrates were transferred to a container until the completion of solvent evaporation at room temperature.The characterisation of lignin in the knot samples was performed using the Klason Lignin degradative method and spectroscopic methods: 13C Nuclear Magnetic Resonance and Fourier transform infrared. Additionally, to corroborate or compare the results obtained in the tests mentioned above, Wiesner, Mäule and Fluorescence histochemical tests were used.Klason Lignin (acid-insoluble lignin) was determined using approximately 300mg of extractive-free material, transferred to a test tube with the slow addition of 3mL of H2SO4 – 72%. The material was stirred for 1 hour at a temperature of between 25 and 30°C, transferred to a 250mL flask and diluted in a 15% H2SO4 solution, in addition to 84mL of distilled water. This was later left at reflux for 4 hours, and then left to rest. After resting, the residue was washed with 500mL of distilled hot water in a synthesised plate funnel, with the aid of a vacuum. The material retained on the filter was dried in an oven at 105°C until the weight remained constant (Abreu et al., 2006). The insoluble lignin sample was represented as a percentage, according to Equation 1.
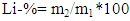 | (1) |
3. Results
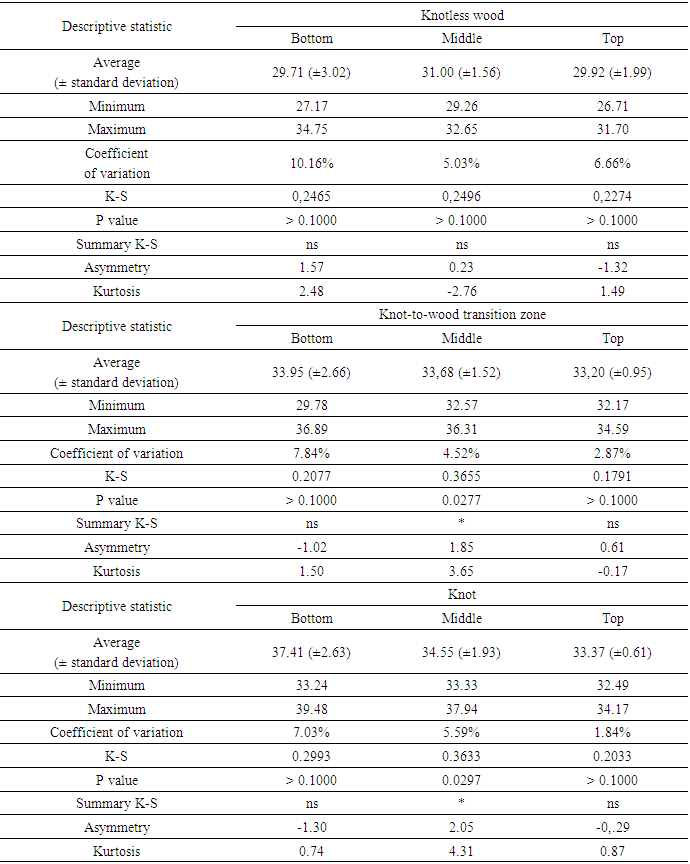
- The percentage data of Klason lignin obtained from the wood of Pinus elliottii var. elliottii Engelm, present a normal or relatively normal distribution, with the exception of the “middle” section of the trunk, bothin the “knot-to-wood transition zone” and “knot”. The variation coefficients presented low percentages, suggesting a relative numerical consistency of the obtained averages (29-37%), as shown in Table 1.
|
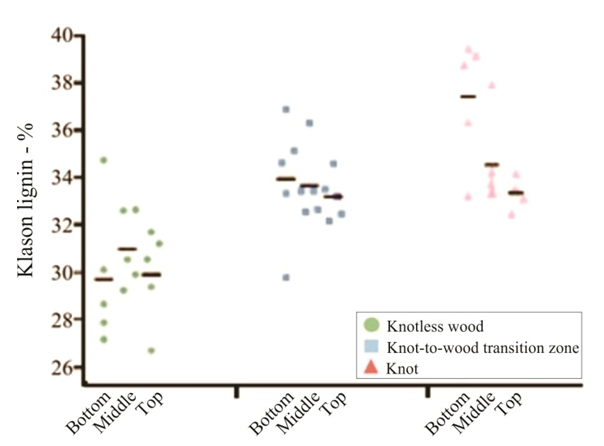 | Figure 1. Percentage of Klason lignin from Pinus elliottiivar. elliottii, considering the conditions of “knotless wood”, “knot-to-wood transition” and “knot” and the position on the trunk (bottom, middle and top). Black lines represent the averages in each situation |
|
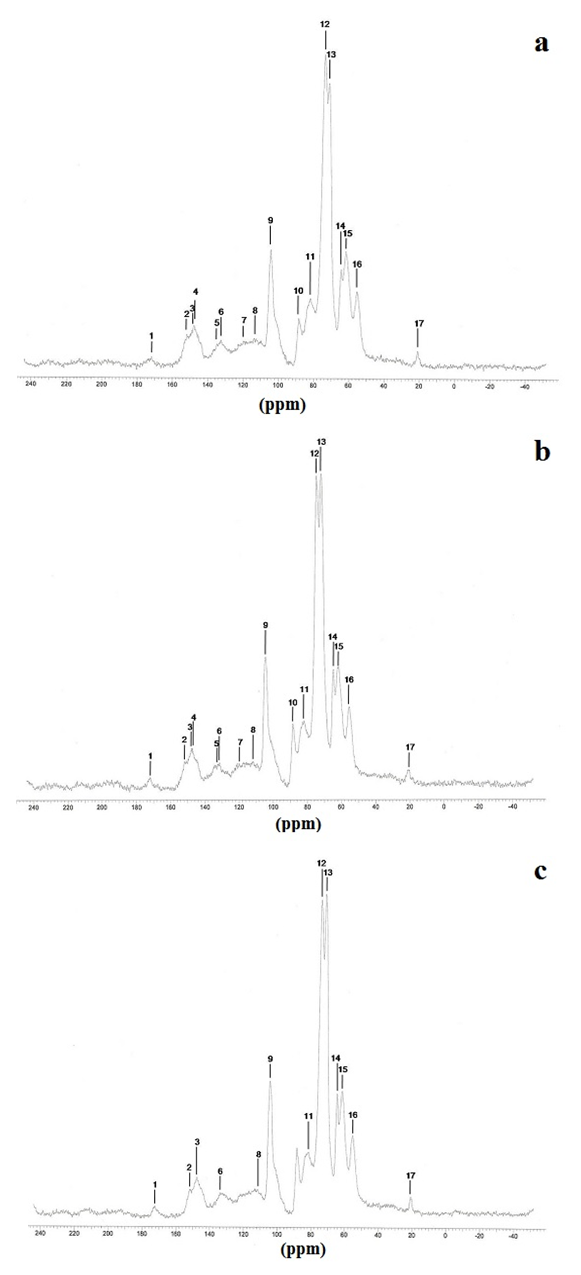 | Figure 2. (a): 13C CP/MAS NMRspectra of the wood without extractives, from the knot at the bottom region (25% of bole length); (b) middle region (50% of bole length) and (c): the top region (100% commercial height) of Pinus elliottii var. elliottii |
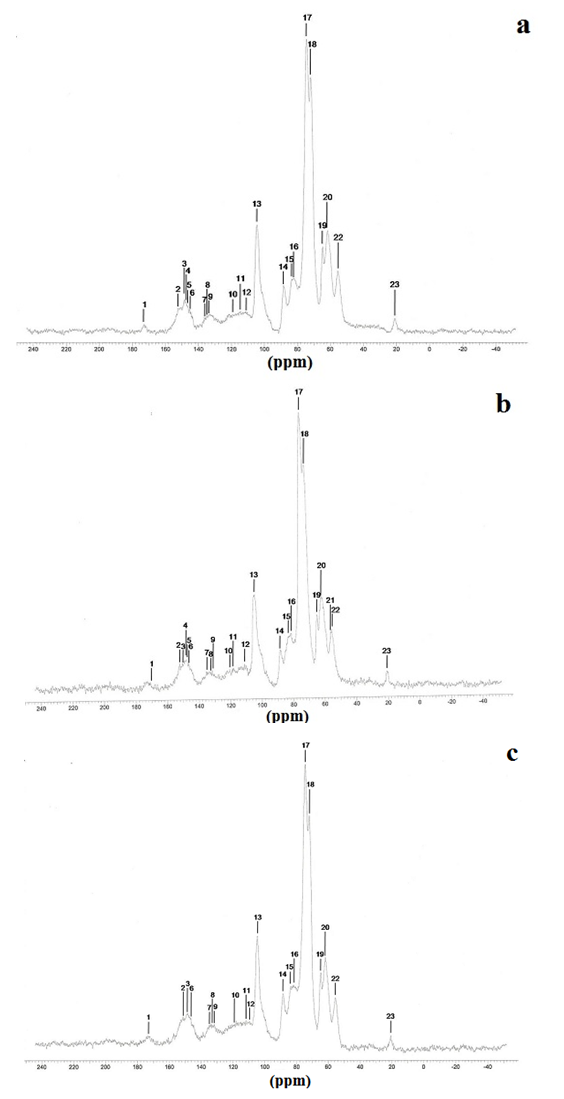 | Figure 3. (a): 13C NMR spectra from knotlesswood at the bottom (25% of bole height); (b) middle region (50% of bole height) and (c): top region (100% of bole height) of Pinus elliottii, var. elliottii Engelm |
|
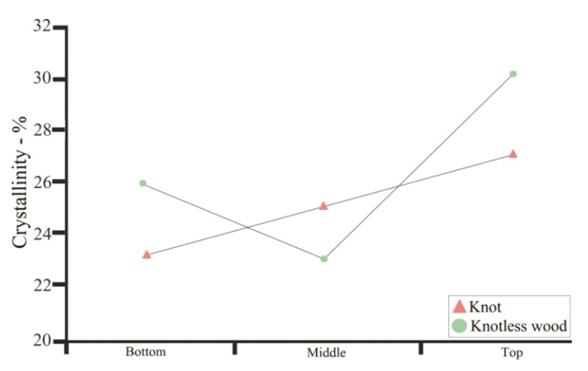 | Figure 4. Percentage of cellulose crystallinity in knotted wood region and knotless wood, along the trunk of Pinus elliottii, var. elliottii Engelm |
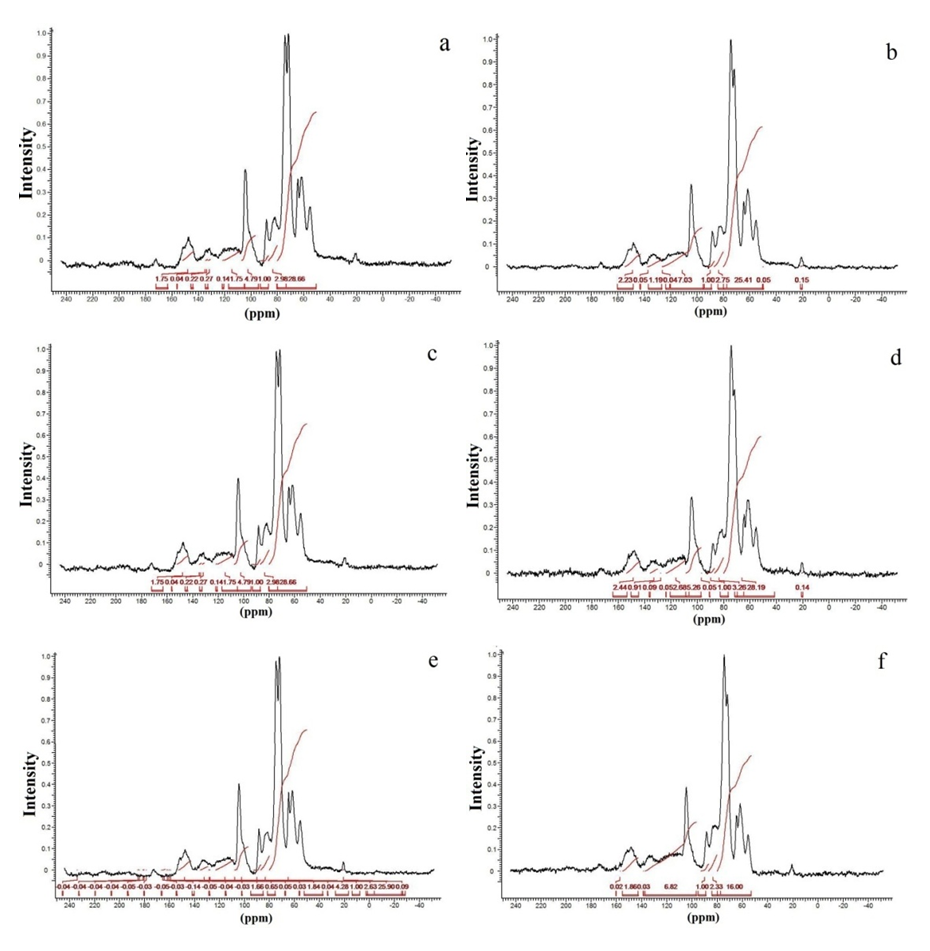 | Figure 5. 13C NMR CP/MAS spectra of extractive-free wood, integrated in the region between (80-97 ppm) from the bottom region (25% of bole height) of the knot (a) and normal (b), from the middle region of the trunk (50% commercial height) for the knot (c) and normal (d) and from the top region (100% commercial height) of the knot (e) and normal (f) in Pinus elliottii, var. elliottii Engelm |
|
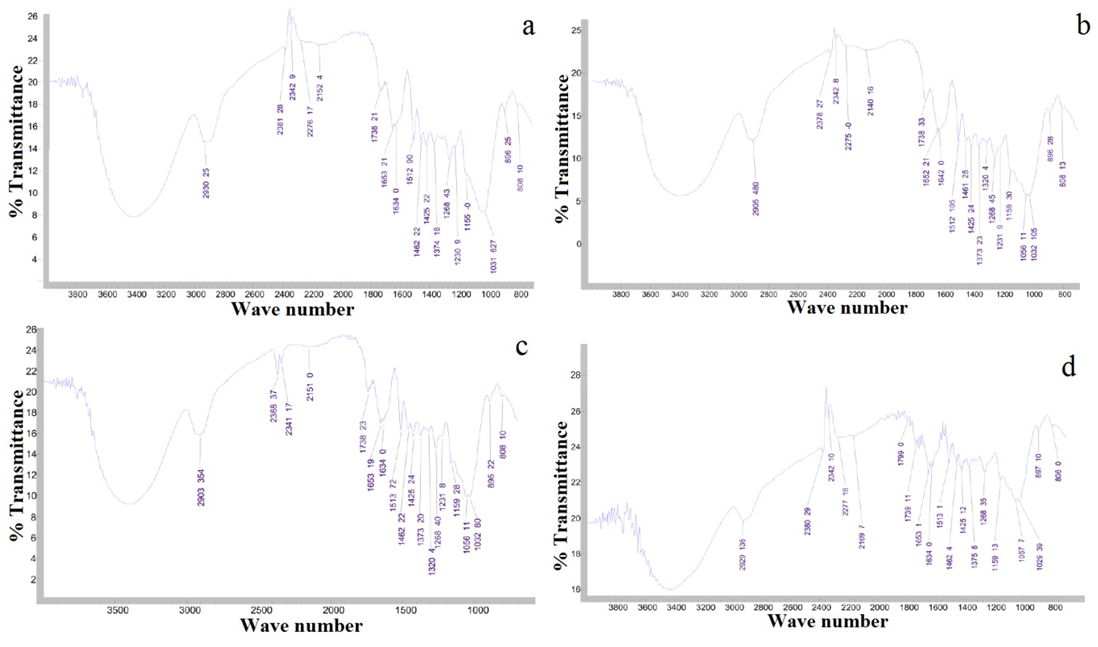 | Figure 6. (a): Fourier transform infrared spectrum in the bottom region of extractive-free knotted wood; (b) in the middle region of extractive-free knotted wood; (c): in the top region of extractive-free knotted wood; (d): in the bottom region of extractive-free knotless wood; (e): in the middle region of extractive-free knotless wood and (f) in the top region of extractive-free knotless wood of Pinus elliottii var. elliottii Engelm |
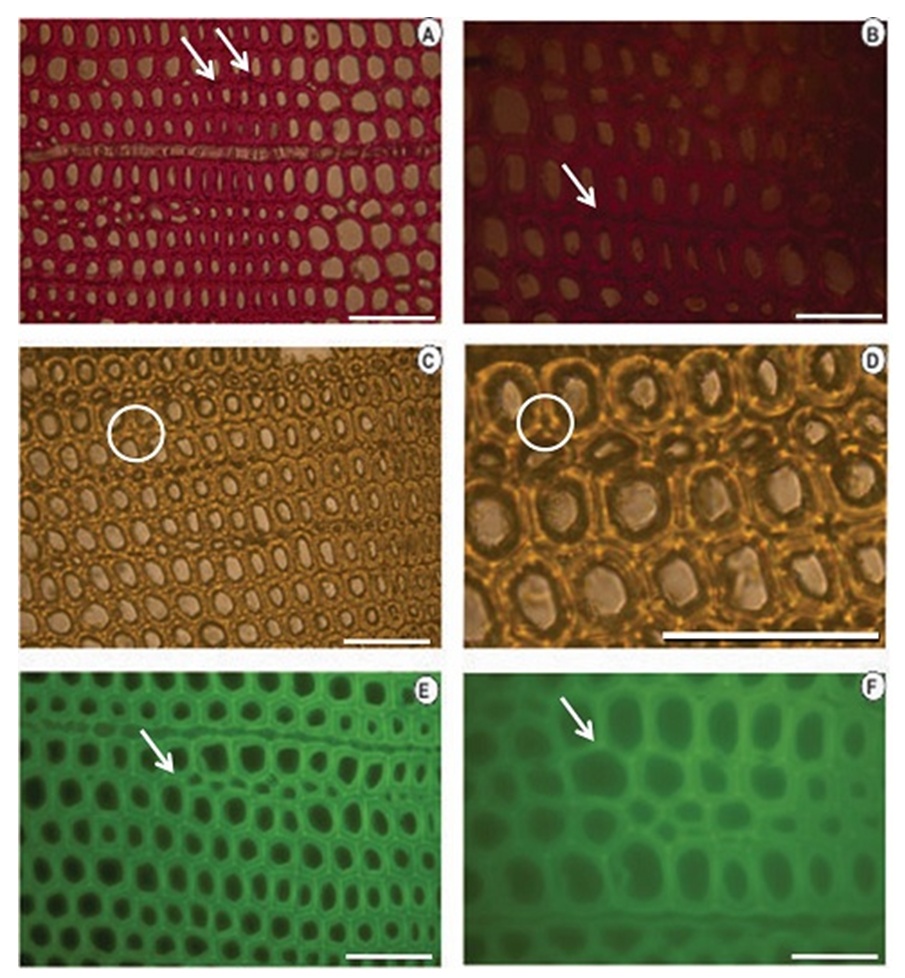 | Figure 7. Histochemical tests applied to a cross-section of knotless wood from Pinus elliottii var. elliottii Engelm (A, B): Wiesener test showing the presence of more aldehyde lignin (darker colour) at the edges at the start of the lignification process. (C, D): Maüle test showing the presence of type G lignin. (E, F): Lignin autofluorescence showing the start of the lignification process at the edges (SETAS) of the cells. Bars (A, C, D, E) = 20μm; (B, F) = 100 μm |
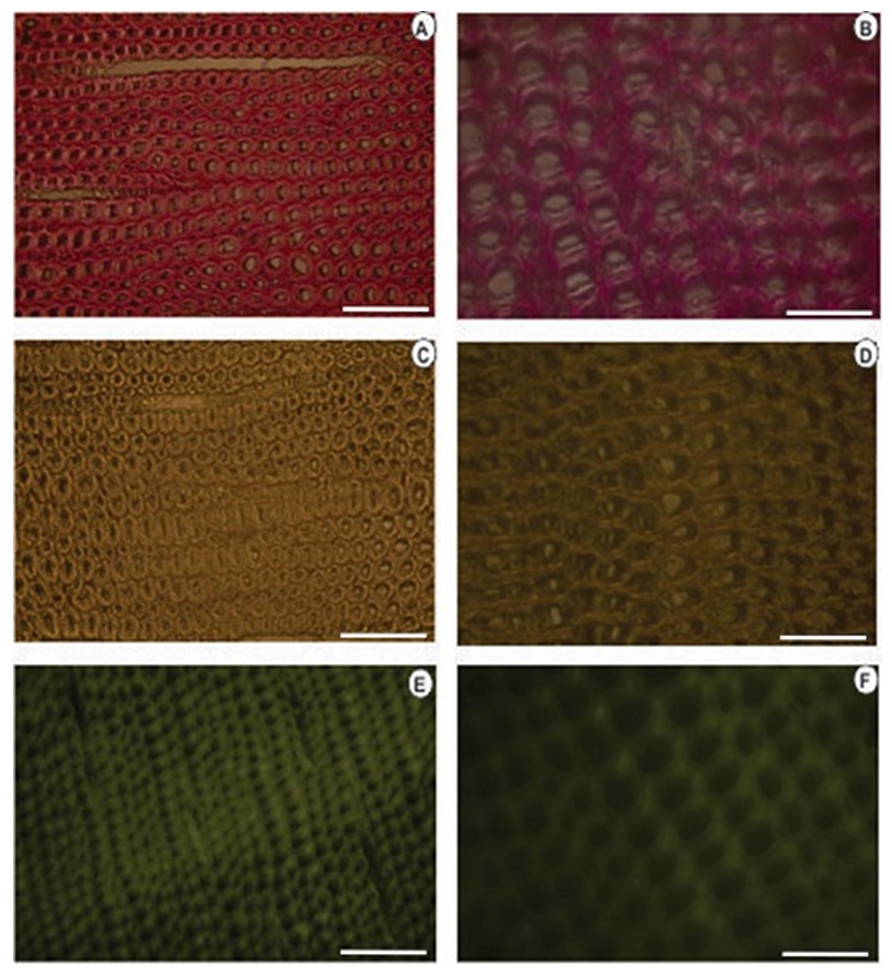 | Figure 8. Histochemical tests applied to the cross-section of knotted Pinus elliottii var. elliottii Engelm wood (A, B): Wiesener test showing less aldehyde lignin (lighter colour compared to the colour of knotless wood). (C, D): Maüle test (E, F): Lignin autofluorescence. Bars: (A, C, E) = 20μm; (B, D and F) = 100 |
4. Discussion
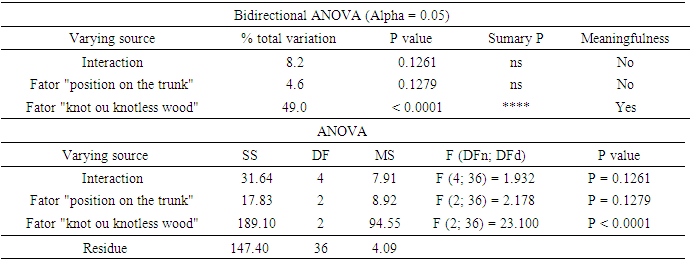
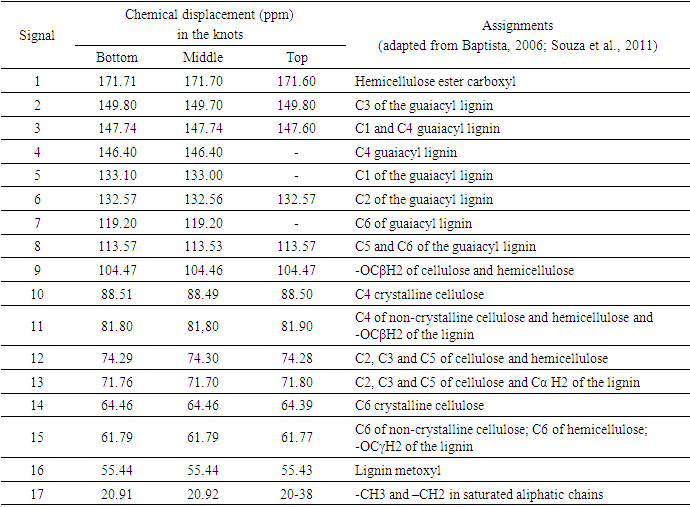
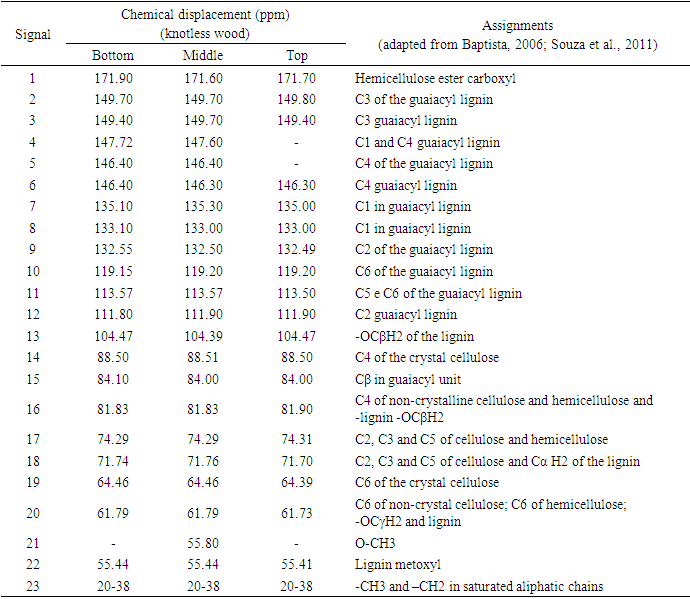
- For some authors, the lignin content reported for coniferous wood ranged from 25-35% (Sansígolo and Barreiros, 1998; Carvalho et al., 2009). Analysing the percentages found in Pinus elliottii var. elliottii Engelm in this study, both for knotless wood and transition wood, the values for almost all individuals correspond to the expected range for coniferous wood. Studies have found values of 28% of insoluble lignin for the same species (Balloni, 2009). In knotted wood, the maximum percentage values of Klason lignin represent 39% and 37%. In studies with samples of 30 Norway Spruce knots (Willför et al., 2003c), variations between 6-24% were found for lignin content.In this study, considering the radial orientation, the samples were taken horizontally more externally. Authors claim that there is a gradient of lignin concentration in knots, with a higher percentage occurring in the knotted portions inserted inside the trunk, progressively decreasing to a level below 1% as the knot is projected between 10-20 cm out of the trunk (Willför et al., 2003c). This suggests that, for knotted Pinus elliottii var. elliottii Engelm wood, the percentage of Klason lignin could be even higher than found here.Compared to Araucaria angustifolia (Bertol.) Kuntze and Pinus silvestre L., the percentage of lignin for Pinus elliottii var. elliottii was lower, but confirms the percentage increase of lignin in the knot (Hillis and Inoue, 1968; Anderegg and Rowe, 1974), compared to the tissue adjacent to the knot and the tissue of the knotless wood. According to the authors, in A. angustifolia the percentage of lignin in knots is up to 20% higher than in the wood adjacent to the knots. This may be related, according to Willför et al. (2003a), to the oligolignans formed at an early age of the tree or knot and mainly to the lignans produced as the knot ages.Other studies (Willför et al., 2003a; (Willför et al., 2003b) found that P. silvestris knotted wood (predominant species in Europe) contains from 0.4-2.9% more lignin than the wood adjacent to the knot. Other authors (Phelan et al., 2009) stated that the content of phenolic substances varies significantly, not only between species, but between the knots of the same tree. The scarcity of literature that justifies the variation of lignin content exclusively in knots, limits the comparison of the results obtained in this study with the variation of lignin observed in compression wood.The similarity of the lignin concentration found in Pinus elliottii var. elliottii for the three regions (bottom, middle and top), can be explained by the reduced number of individual samples (five), since for the series constituted by the set of average samples converging to the population average, the sample size (n) must be significantly large, based on the Central Limit Theorem (Gotelli and Ellison, 2011; Rodrigues, 2011). In a study of more than 30 samples of knots from seven Norwegian spruce trees, the authors demonstrated a percentage of 6 to 24% more lignin (Willför et al., 2003c), while the other authors (Holmbom et al., 2003), studying more than 50 individuals and showed that the knots could contain higher quantities of polyphenols than the wood next to the knot, with these amounts reaching up to 100 times more for some species. In an evaluation of knot extracts from Pinus sitchensis (Bong.) Carrière and Pinus banksiana Lamb. (Phelan et al., 2009), the authors verified that there was no similarity in the lignin values between species, with the P. sitchensis knot extract presenting content that was three times higher. Other authors did not evaluate the variation in the lignin content of knots along the trunk.Regarding the 13C magnetic resonance spectra of wood from the “bottom knot”, “middle knot” and “top knot” regions, the comparison of values obtained with the results of other authors (Landucci et al., 1998; Souza et al., 2011) showed that spectra signals of 13C NMR from P. elliottii, var. elliottii can be divided into three main types: acetyl groups (168-171 ppm); aromatic carbons, which can be divided into quaternary (125-160 ppm) and methylene carbon atoms (110-125 ppm) and signals from side chain carbon atoms (50-90 ppm). Despite the similarity of the 13C spectrum signals for the three regions, differences in absorption intensity relativeto each signal were observed when comparing signals obtained from the bottom knot region with the carbon signals from the middle knot and top knot regions. Although the absorptions may reveal errors due to contributions from the 1H nucleus, they are minimised in their amplitude due to the combination of radiation between the 13C and 1H nuclei.As such, the crystallinity indices of cellulose from different regions of wood were estimated. This estimate was based on dividing the areas of crystalline and non-crystalline C4 signals between 80-93 ppm. The determination was calculated based on dividing the area of the crystalline cellulose C4 signal (87-90 ppm) by the sum of the C4 signals between in 80-93ppm (Park et al., 2010).Signals characteristic of C2, C3 and C5 carbons and of cellulose and hemicellulose – signal 12 (Table 3) with the same intensity, were obtained from the bottom knot, middle knot and top knot regions. Signals characteristic of C- α in β-O-4 G units (guaiacyl lignin) were similar between the spectra obtained from the middle and top regions of knotted wood – signal 13 (Table 3) and Figures 2b and 2c, but different from signals observed in the spectra obtained from the bottom knot wood (Figure 2a).These signals (C-α in units β-O-4-G) were similar to the signals obtained from the knotless wood from the three analysed regions (bottom, middle, top). The C2, C3 and C5 regions of cellulose and C-α H2 lignin presented higher intensities in the middle and top samples compared to bottom samples. C6 signals, characteristic of crystalline cellulose, were observed in all the knot region spectra – signal 19 (Table 3), but lower signal intensity and greater signal width were observed in the spectra of the bottom knot region (Figure 2a).While the percentage of Klason lignin did not differ when compared longitudinally, the percentage of cellulose crystallinity showed significant variation. In the knot region there was an increasing linear variation in cellulose crystallinity, with the bottom knot wood presenting the lowest percentages (23%) followed by the middle knot wood (27%) and the top knot wood presenting the highest cellulose crystallinity (30%).Comparing the “bottom knot” and “knotless wood bottom” conditions separately, the percentage of cellulose crystallinity washigher in “knotless wood bottom”. The isolated comparison between the “middle knot” and “knotless middle” revealed the inversion of these values, where the “middle knot” presented more molecular crystallinity. In the evaluation of the top regions, the condition “knotless top” presented the highest percentage of molecular cellulose crystallinity. Compared to each other, it is possible to identify the same percentage of cellulose crystallinity between the “bottom knot” and “knotless middle” conditions.In the analysis of 13C NMR CP/MAS spectra, the similarity of signals found between knotted and knotless wood samples and the comparison of the results with values found in the literature, suggest the same chemical composition between regions. This presupposes that in all the samples, the physiological process quantitatively maintained the same ratio (Souza et al., 2011). Broad signals, which indicate high heterogeneity and altered chemical structure (Froass et al., 1996), were discretely observed in knotless wood spectra.The comparison of 13C NMR data with values described in the literature (Lago and Roque, 2009), suggest that the lower quantity of guaiacyl lignin signals observed in the knot sample spectra is related to the interference of other substances, observed in the knot samples spectra is related to the interference of other substances, where the functional groups are obscured by the complexity of natural polymers.Lignin isolated from most hard woods is essentially pure guaiacyl lignin (Obst and Landucci, 1986) but the difficult removalof traces of carbohydrates and foreign components, especially if they are covalently connected to the lignin polymer, interferes with the visualisation of the corresponding signal (Landucci et al., 1998). The heterogeneity of signal intensity, especially for signals characteristic of cellulose, hemicellulose and cellulose molecule crystallinity, although subtle, also indicate the interference of other chemical elements.Comparing the Klason lignin percentage graphs with thecellulose crystallinity percentage graph, it is possible to infer the existence of a correlation between the lignin content and cellulose molecule crystallinity. Other authors (Donaldson and Knox, 2012) claim thatthere are consistent correlations between hemicellulose components (polysaccharides containing galactose, mannose and xylose) and cell wall lignification, and although less than 5% of data variation in the percentage of lignin in the bottom, middle and top regions of P. elliotti var elliotti was explained by the variable position on the trunk, the isolated values of these percentages suggest increasing variation along the trunk.The lack of similarity presented for the percentages of lignin observed between knot regions and the adjacent regions, corroborates the thesis of the existence of differentiated patterns in the process of formation, differentiation, and maturation of knotted wood cells. The variation in cellulose crystallinity showed an increasing percentage as trunk height increased. This variation can be attributed to the influence of hormonal factors. The closer to the apex, the greater the influence of AIA (auxin) on the formation of tracheal elements and the greater the number of cells in the process of cellular division, the lower the increments of lignin disposition or secondary chemical components (Aloni, 2023).For cell formation, several cellulose chains unite through hydrogenic connections to form microfibrils, which can be associated with other macromolecules through their -OH groupings. At the start of microfibril formation, cellulose is presented in its crystalline form. As the microfibril increases in size, with the deposition of more cellulose chains, it starts to suffer from the interference of localised defects, contributing to the formation of a greater amorphous region of cellulose (Oliveira, 2009). Since the cells of the knot top region are experiencing a greater rate of division, the cellulose chains responsible for the formation of microfibrils do not associate with other macromolecules, remaining mainly crystalline. The decreasing polar flow of auxin, from the cauline apex, induces the differentiation of tracheal elements, promoting more cell division and an increase in the diameter of elements (Aloni, 1987; Aloni and Peterson, 1997; Evert, 2013). However, this gradient is also responsible for the decrease in element density, in the direction of leaves to roots (Aloni and Zimmermann, 1983). As they are closer to the physiologically more active zones of AIA production (young leaves), the branch/trunk intercession points (knots) are more susceptible to the action of this hormone.Regarding the induction of tracheal differentiation by decreasing auxin, this may be associated with hormonal balance, with an increase in cytokinin regulating cell division. As well as the reduced concentration of the inhibitory hormone abscisic acid and the increase in day length, inducing greater exchange activity (Galston and Davies, 1972; Taiz et al., 2021). According to the authors, the plant's response to auxin depends both on the nature of the tissue and on the concentration of the substance present.The difference in crystallinity behaviour observed in the knotless wood region, where the wood region at the bottom presented more crystallinity than wood in the middle of the trunk, can be explained by the heterogeneity of growth, thickening of anatomical elements and hormonal action as described in the literature.In the histochemical tests, the lignification of the cell wall was divergent between knotted and knotless wood. Studies have shown that aldehyde structures are constructed during the initial stages of xylem cell wall lignification and that the Wiesener staining test can be used for the specific detection of these structures contained in lignin (Pomar et al., 2002). In a study using the stem sections of Zinnia elegans L. treated with the Wiesener and Maüle test (Barceló et al., 2000), the authors observed that the colour of sections stained with phloroglucinol-HCL (Wiesener) extended beyond young xylem cell differentiation, while staining with Maüle reaction was restricted to already differentiated xylem cells.Compared to the results in the literature (Fukushima and Terashima, 1991) and supported by the results of the chemical lignin analyses, where lower lignin contents were obtained for knotless wood, it is possible to infer that the knotless wood and knotted wood tissues, although close, present different phases of lignification. Since the process is mirrored through the secondary wall towards the lumen (Donaldson, 2001) and is controlled to a significant extent for individual tracheids (Donaldson, 1994), the brown-gold colour observed in the cells of knotless wood using the Maüle test suggests a lower level of lignification maturation and a lower lignin content in the cell wall. Studying reaction tissue formation in Medicago sativa L. (Patten et al., 2007), the authors found that the percentage of lignin can increase tenfold between the stages of cell wall lignification. These authors observed that the greater the proportion of guaiacyl lignin in the tissue, the more potentialized (dark brown) the Maüle test reaction.The results obtained from the histochemical tests corroborate the results obtained from the 13C NMR, where greater quantities of carbon signals characteristic of guaiacyl lignin were attained from the spectra of the knotless wood regions. In the fluorescence test, it was possible to observe lignin surrounding the cells of knotless wood samples.Based on Emonet and Hay (2022), the local lignin deposition surrounding the cells of the knotless wood cells is a common mechanism that follows precise patterns, although with distinct genetic basis in different cell types with functions in different tissue contexts. Mainly for tracheal elements, as they depend on a lignified secondary cell wall to provide mechanical strength, rigidity and hydrophobicity.The vast majority of scientific research on macromolecular lignin, its initiation sites and cell wall lignification are limited to the investigation of these processes in wood cells whose growth is normal or at best, compression and rection cells (Whetten and Sederoff, 1995; Donaldson, 2001; Grabber, 2005; Donaldson and Knox, 2012). Given the high variety found in the wood, either longitudinally or radially, between the anatomical elements and even between the chemical elements (Zimmermann and Potter, 1982; Muniz, 1993; Ballarin and Palma, 2003; Silva et al., 2005) it can be assumed that the results obtained in such research express, with a certain approximation, but not certainty, the dynamics of anatomical formation and chemical composition of cells that compose knotted wood.The differentiated lignification between normal wood and compression wood, is explained by the chemical variation of the cell wall (Donaldson, 2001), where the middle lamella of normally growing wood cells is more lignified than the secondary wall. In compression wood, this pattern is significantly modified, with a reduction in average lignification in the middle lamella and an increase in the lignification of the outer secondary wall (Donaldson, 2001).The auxin concentration gradient, proposed by some authors (Aloni and Zimmermann, 1983), also allows lower lignin deposition and secondary chemical elements in the cells of knotless samples, both at the bottom and middle bole, since the low auxin concentrations induce slow cell differentiation and that the cells of these regions are more distant from the auxin producing regions, which limits the action of the hormone (Shimoyama, 2005; Evert, 2013).
5. Conclusions
- There is anincreasing gradient of lignin percentage between knotless wood, transition wood and knotted wood.In knotted wood tracheids, lignification is at a more advanced stage of maturation than the knotless wood trachids and the lignin present in the knotless wood tracheids are more aldehydic than lignin in knotted tracheids.The development dynamics between the anatomical elements of knotless wood and knotted wood showed different patterns. It is possible to conjecture that such characteristics interfere with the resistance of wood in these regions along the trunk.Crystalline cellulose signalin “knotless wood” were less intense compared to signals in the middle knot and topknot regions. The bottom region of wood showed higher crystallinity than the wood in the middle trunk and lower than the top region.The lignin structure in the regions of knotless and knotted wood was different, despite the proximity of these regions to each other.
References
| [1] | Oliveira, J. T. S. (1997). Caracterização da madeira de eucalipto para construção civil. 429 p. |
| [2] | Silva, J. C. (2002). Caracterização da madeira de Eucalyptus grandis Hill ex. Maiden, de diferentes idades, visando a sua utilização na indústria moveleira, 160 p. |
| [3] | Gatto D. A., Calegari, L., Santini, E. J., Stangerlin, D.M., Trevisan, R. and Oliveira, R. S. (2008). Propriedades da madeira de Pinus elliottii Engelm submetida a diferentes temperaturas de secagem. Revista Cerne, 14(3): 220-226. |
| [4] | Britez, C. A. and Nogueira, V. (2006). Inter-relação entre as propriedades e a microestrutura das madeiras. 25 p. |
| [5] | Abreu, H., Maêda, J., Latorraca, J., Pereira, R., Monteiro, M.B., Abreu, F. and Carmo, J. (2004). Proposta de Modificação da Biossíntese da Lignina como Estratégia para Correção de Defeitos em Madeiras. Silva Lusitana, 11: 217 - 225. |
| [6] | Deus, D. A. D., Zaú, A. S., Muniz, G. I. B. D., Nisgoski, S., Abreu, H. D. S. and Gama, D. C. (2022). Lignina: uma importante tecnologia química da madeira. E-Acadêmica, 3(3): e7233391-e7233391. |
| [7] | Patten, A. N., Jourdes, M., Brown, E. E., Laborie, M-P., Davin, L. B. (2007). Lewis, N. G. Reaction tissue formation and stem tensile modulus properties in wild-type and p-coumarate-3-hydroxylase down regulated lines of alfalfa, Medicago sativa (Fabaceae). American Journal of Botany, 94(6): 912-925. |
| [8] | [8] FENGEL, D.; WEGENER, G. Wood: chemistry, ultrastructure, reactions. Verlag Kessel. Berlin. 613 p. 2003. |
| [9] | Klock, U., Muniz, G. I. B., Hernandez, J. A. and Andrade, A.S. (2005). Apostila de química da madeira. |
| [10] | Wilkie, J. S. (1961). Carl Nägeli and the fine structure of living matter. Nature, 190: 1145-1150. |
| [11] | Nishiyama, Y., Langan, P. and Chanzy, H. (2002). Crystal structure and hydrogen-bonding system in cellulose iβ from synchrotron X-ray and neutron fiber diffraction. Journal of the American Chemical Society, 124(31): 9074-9082. |
| [12] | Oliveira, R. M. (2009). Utilização de técnicas de caracterização de superfícies de madeiras tratadas termicamente, 123p. |
| [13] | Mokfienski, A. (2004). Importância relativa da densidade básica e da constituição química de madeira de Eucalyptus spp. no rendimento, branqueabilidade da qualidade da polpa Kraft, 136 p. |
| [14] | Stam, A. J. (1964). Wood and cellulose science. New York. Ronald Press. |
| [15] | Thygesen, A., Oddershede, J., Lilholt, H., Thomsen, A. B. and Stahl, K. (2005). On the determination of crystallinity and cellulose content in plant fibres. Cellulose, 12(6): 563-576. |
| [16] | Dufresne, A. and Belgacem, M. N. (2013). Cellulose-reinforced composites: from micro-to nanoscale. Polímeros, 23(3): 277-286. |
| [17] | Klinke, H. B., Lilholt, H., Toftegaard, H., Andersen, T. L., Schmidt, A. S. and Thomsen, A. B. (2000). Wood and plant fibre reinforced polypropylene composites. In: Proceedings, Biomass for energy and industry: 1st World conference and technology exhibition, Sevilla, 2: 1082-1085. |
| [18] | Marvila, M. T., Rocha, H. A., Azevedo, A. R. G. D., Colorado, H. A., Zapata, J. F. and Vieira, C. M. F. (2021). Use of natural vegetable fibers in cementitious composites: Concepts and applications. Innovative Infrastructure Solutions, 6: 1-24. |
| [19] | Lahr, F. A., Nogueira, M. C., Araujo, V. A. D., Vasconcelos, J. S. and Christoforo, A. L. (2018). Wood utilization of Eucalyptus grandis in structural elements: densities and mechanical properties. Engenharia Agrícola, 38: 642-647. |
| [20] | Browning, B. L. (1967). Methods of wood chemistry – Interscience Publishers – New York, 2: 800 p. |
| [21] | Abreu, H. S., Carvalho, A. M., Monteiro, M. B. O., Pereira, R. P. W., Silva, H. R., Souza, K. C. A., Amparado, K. F. and Chalita, D. B. (2006). Métodos de análise em química da madeira. Floresta e Ambiente, Série técnica: 01-20. |
| [22] | Park, S., Baker, J. O., Himmel, M. E., Parilla, P. A. and Johnson; D. K. (2010). Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance. Biotechnology for Biofuels, 3: 1-10. |
| [23] | Morais, S. A. L., Nascimento, E. A. and Melo, D. C. (2005). Análise da madeira de Pinus oocarpa parte I – estudo dos constituintes macromoleculares e extrativos voláteis. Árvore, 29(3): 46-470. |
| [24] | Vazquez-Cooz, I. and Meyer, R.W. (2002). A differential staining method to identify lignified and unlignified tissues. Biotechnic and Histochemistry, 77(5-6): 277-282. |
| [25] | Lin, S. Y. and Dence, C. W. (1992). Methods in lignin chemistry. Berlim: Spring-Verlag, 568 p. |
| [26] | Hori, R. and Sugiyama, J. (2003). A combined FT-IR microscopy and principal component analysis on softwood cell walls. Carbohydrate Polymers, 52(4): 449-453. |
| [27] | Sansígolo, C. A. and Barreiros, R. M. (1998). Qualidade da madeira de Pinus caribaea var. hondurensis para produção de celulose kraft. In: Congresso Anual de Celulose e Papel Da ABTCP, São Paulo. Anais..., 31: 417- 429. |
| [28] | Carvalho, W., Canilha, L., Ferraz, A. and Milagres, A. M. F. (2009). Uma visão sobre a estrutura, composição e biodegradação da madeira. Química Nova, 32(8): 2191-2195. |
| [29] | Balloni, C. J. V. (2009). Caracterização física e química da madeira de Pinus elliottii. 42 p. |
| [30] | Willför, S., Nisula, L., Hemming, J., Reunanen, M. and Holmbom, B. R. (2003c). Bioactive phenolic substances in industrially important tree species. Part 1: Knots and stem wood of different spruce species. Holzforschung, 58(4): 335-344. |
| [31] | Hillis, W. E. and Inoue, T. (1968). The formation of polyphenols in trees-IV: The polyphenols formed in Pinus radiate after Sirex attack. Phytochemistry, 7(1): 13-22. |
| [32] | Anderegg, R. J. and Rowe, J. W. (1974). Lignans, the major component of resin from Araucaria angustifolia knots. Holzforschung, 28(5): 171-175. |
| [33] | Willför, S., Hemming, J., Reunanen, M., Eckerman, C. and Holmbom, B. R. (2003a). Phenolic and lipophilic extractives in Scots pine knots and stem wood. Holzforschung, 57(1): 27-36. |
| [34] | Willför, S., Hemming, J., Reunanen, M. and Holmbom, B. (2003b). Phenolic and lipophilic extractives in Scots pine knots and stem wood. Holzforschung, 57(4): 359-372. |
| [35] | Phelan, M., Aherne, S. A., Wong, A. and O’brien, N. M. (2009). Bioactive properties of wood knot extracts on cultured human cells. Journal of Medicinal Food, 12(6): 1245-1251. |
| [36] | Gotelli, N. J. and Ellison, A. M. (2011). Princípios de estatística em ecologia. Porto Alegre: Artmed. 547p. |
| [37] | Rodrigues, C. K. Um breve estudo sobre a abordagem do teorema central do limite nos livros-texto. In: XIII Conferência Interamericana de Educação Matemática, 2011, Recife. Anais... Recife, 2011. |
| [38] | Holmbom, B. R., Eckerman, C., Eklund, P., Hemming, J., Nisula, L., Reunanen, M., Sjöholm, R., Sundberg, A., Sundberg, K. and Willför, S. (2003). Knots in trees – A new rich source of lignans. Phytochemistry Reviews, 2: 331-340. |
| [39] | Souza, N. D., Abreu, H. S., Elias, T. F., Latorraca, J. F. L. and Maeda, J. M. (2011). Dados de carbono molecular do extrato ciclo-hexano da madeira de Eucalyptus urophylla S. T. Blacke por RMN de 13C. Floresta e Ambiente, 18(2): 186-197. |
| [40] | Landucci, L. L., Ralph, S. A. and Ammel, K. E. (1998). 13C NMR characterization of guaiacyl, guaiacyl/syringyl and syringyl dehydrogenation polymers. Holzforschung, 52(2): 160-170. |
| [41] | Froass, P. M., Ragauskas, A. J. and Jiang, J.-Er. (1996). Chemical structure of residual lignin from kraft pulp. Journal of Wood Chemistry and Technology, 16(4): 347-365. |
| [42] | Lago, J. H. G. and Roque, N. F. (2009). Estudo fitoquímico da madeira de Guarea macrophylla (meliaceae). Química Nova, 32(9): 2351-2354. |
| [43] | Obst, J. R. and Landucci, L. L. (1986). Quantitative 13C NMR of lignins – methoxyl: aryl ratio. Holzforschung, 40(suppl.): 87-92. |
| [44] | Donaldson, L. A. and Knox, J. P. (2012). Localization of cell wall polysaccharides in normal and compression wood of Radiata Pine: relationships with lignification and microfibril orientation. Plant Physiology, 158(2): 642-653. |
| [45] | Aloni, R. (2013). The role of hormones in controlling vascular differentiation. Plant Cell Monographs, 20: 99-139. |
| [46] | Aloni, R. (1987). Differentiation of vascular tissues. Plant Physiology, 38: 179-204. |
| [47] | Aloni R. and Peterson C. A. (1997). Auxin promotes dormancy callose removal from the phloem of Magnolia kobus and callose accumulation and early wood vessel differentiation in Quercus robur. Journal of Plant Research, 110(1): 37-44. |
| [48] | Evert, R. F. Anatomia das plantas de Esau: meristemas, células e tecidos do corpo da planta: sua estrutura, função e desenvolvimento. Blucher, 726 p. 2013. |
| [49] | Aloni, R. and Zimmermann, M. H. (1983). The control of vessel size and density along the plant axis - A new hypothesis. Differentiation, 24(1-3): 203-208. |
| [50] | Pomar, F., Merino, F. and Ros Barceló, A. (2002). O-4-Linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol-HCl) reaction. Protoplasma, 220: 17-28. |
| [51] | Barceló, A. R., Pomar, F. and Pedreño, M. A. (2000). Competitive inhibitor-dissected histochemistry of the peroxidase responsible for syringyl lignin biosynthesis in Z. elegans xylem. Australian Journal of Plant Physiology, 27: 1101-1107. |
| [52] | Fukushima K. and Terashima N. (1991). Heterogeneity in formation of lignin. Wood Science and Technology, 25(5): 371-381. |
| [53] | Donaldson, L. A. (2001). Lignification and lignin topochemistry — an ultrastructural view. Phytochemistry, 57(6): 859–873. |
| [54] | Donaldson, L. A (1994). Mechanical constraints on lignin deposition during lignifications. Wood Science and Technology, 28(2): 111-118. |
| [55] | Whetten, R. and Sederoff, R. (1995). Lignin Biosynthesis. Plant Cell, 7(7): 1001-1013. |
| [56] | Grabber, J. H. (2005). How do lignin composition, structure, and cross-linking affect degradability? A review of cell wall model studies. Science Society of America, 45(3): 820-831. |
| [57] | Silva, J. C., Matos, J. L. M., Oliveira, J. T. and Evangelista, W. V. (2005). Influência da idade e da posição ao longo do tronco na composição química da madeira de Eucalyptus grandis Hill ex. Maiden. Revista Árvore, 29(3): 455-460. |
| [58] | Ballarin, A. W. and Palma, H. A. L. (2003). Propriedades de resistência e rigidez da madeira juvenil e adulta de Pinus taeda L. Árvore, 27(3): 371-380. |
| [59] | Zimmermann, M. H.; D. Potter. (1982). Vessel-length distribuition in branches, stem, and roots of Acer rubrum L. IAWA, 3(2); 103-109. |
| [60] | Muñiz, G. I. B. (1993). Caracterização e Desenvolvimento de Modelos para Estimar as Propriedades e o Comportamento na Secagem da Madeira de Pinus elliottii Engelm. e Pinus taeda L., 252p. |
| [61] | Shimoyama, V. R. S. (2005). Estimativas de propriedades da madeira de Pinus taeda através do método não destrutivo emissão de ondas de tensão, visando a geração de produtos de alto valor agregado, 151 p. |
| [62] | Emonet, A.; Hay, A. (2022). Development and diversity of lignin patterns. Plant Physiology, 190(1), 31-43. |
| [63] | Galston, A. W.; Davies, P. J. (1972). Mecanismo de controle no desenvolvimento vegetal. Edgard Blucher: São Paulo, 171p. |
| [64] | Taiz, L.; Zeiger, E.; Moller, I. M.; Murphy, A. (2021). Fundamentos de fisiologia vegetal. Artmed: Porto Alegre, 558p. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML