-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2024; 14(1): 18-24
doi:10.5923/j.ijaf.20241401.02
Received: Feb. 8, 2024; Accepted: Feb. 28, 2024; Published: Mar. 22, 2024

Nutrient Digestibility, Rumen Parameters and Microbial Population of WAD Goats Fed Varying Levels of Concentrate Based Diet
Okpara Oghenesuvwe, Ufuoma Godstime Sorhue, Unukevwere Jerome, Onowhakpor Collins
Department of Animal Science, Delta State University Abraka, PMB 1 Abraka, Nigeria
Correspondence to: Ufuoma Godstime Sorhue, Department of Animal Science, Delta State University Abraka, PMB 1 Abraka, Nigeria.
| Email: |  |
Copyright © 2024 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study was conducted to determine effects of feeding varying levels of concentrate diet on nutrient digestibility and rumen fermentation parameters of West African dwarf goats. Forty-five West African dwarf goats aged between 4 and 5months old with mean live weight of 4.70 – 5.0kg were allotted to five dietary treatments with three replicates of nine goats per treatment in a completely randomized design. The compared diets were: T1(fed 3.2% of their bodyweight), T2 (3.4% bodyweight), T3 (3.6% bodyweight), T4 (3.8% bodyweight) and T5 (fed 4.0% bodyweight). A metabolism trial was conducted at the end of the feeding trial to assess the diets on nutrient digestibility after the nutrient intake study of the goats. Rumen fluid was collected at the end of feeding trial to determine rumen fermentation parameters and microbial population. For rumen fermentation characteristics, data were collected on rumen pH, ammonia nitrogen and volatile fatty acids (VFAs) production. Data collected were subjected to one-way Analysis of Variance (ANOVA).Results obtained showed that the digestibility of dry matter, crude protein, ether extract, ash and NFE were influenced (p<0.05) by treatment diets. However, crude fibre digestibility was not influenced (P>0.05) by varying the level of ration. The crude protein digestibility (82.70) was significantly higher (p<0.05) in T1 when compared to other treatments. For rumen fermentation parameters, data were collected on rumen pH, ammonia nitrogen and volatile fatty acids (VFAs) production. The result indicated that all the parameters examined were not significantly (P<0.05) influenced by dietary treatments except for total VFA and acetic acid, which differed significantly across the treatments. There was a significant (P<0.05) increase in value of acetic acid across the treatments at the end of the experiment. The study revealed that feeding West African Dwarf goats concentrate based diet has the potentials of meeting the nutritional needs of the animal without negative effects on the rumen parameters.
Keywords: Rumen parameters, Microbial population, Digestibility, Concentrate diet, WAD goat
Cite this paper: Okpara Oghenesuvwe, Ufuoma Godstime Sorhue, Unukevwere Jerome, Onowhakpor Collins, Nutrient Digestibility, Rumen Parameters and Microbial Population of WAD Goats Fed Varying Levels of Concentrate Based Diet, International Journal of Agriculture and Forestry, Vol. 14 No. 1, 2024, pp. 18-24. doi: 10.5923/j.ijaf.20241401.02.
Article Outline
1. Introduction
- Small ruminants, especially goats, are crucial to the livelihood of small-holder farmers because they can transform inexpensive feed resources into high-value goods (meat, milk, and skin). A good supplier of meat, milk, and other byproducts, goats are one of the most significant, adaptable, and widely distributed livestock animals (Ajibike et al, 2016). They obtain nutrients from the feed, which are necessary for bodily upkeep, growth, and reproduction (protein and energy are the two main nutrients required). The productivity of the animal decreases if the nutrients are not balanced correctly to suit its unique needs for production. Peacock (1996) asserts that appropriate nutrition is a requirement for good health, effective reproduction, high milk yield, rapid growth, and a productive goat production system. However, issues with feed procurement limit the ability to provide good nutrition (Chidebele and Njondjou, 1997). Despite the fact that goats are thought to be superior to other ruminant species in their use of high-fiber, low-quality forage for body production and maintenance (Okpara et al., 2014), an improvement in this potential through improved use of supplements and agricultural wastes could enhance goat productivity.The West African Dwarf (WAD) goat breed is a significant native breed of goat that is well suited to humid and sub humid areas known to be connected with tsetse flies that transmit trypanosomiasis. Ruminant animals are kept on natural grasslands, crop residues, and agro-industrial byproducts as their primary source of nutrients in the majority of tropical countries (AFRC, 1991). As a result, pasture grass and legumes have been promoted for use in the production of small ruminant animals since they make an easy source of feed. In Nigeria, there is a severe feed scarcity that affects goat production, especially during the dry season when supplies are scarce. Poor pasture quality and insufficient nutrient content prevent it from meeting goats' nutritional and maintenance needs (Onyeonaguet al, 2011). The majority of regions where pastures are cultivated with a shortage of traditional feedstuffs experience overgrazing, which exacerbates the situation further. As a result of their stock's delayed growth and irregular weight gain caused by a seasonal imbalance in feeds, goat farmers in Nigeria have had a difficult time making a profit from their animals (Okoruwa and Bamigboye, 2015).The quest for alternative feed sources that might be employed as less expensive available alternatives to grasses has been prompted by the lack of appropriate forage supply to meet the nutrient requirements of goats, particularly during the dry season and the high cost of feedstuffs (Okoruwaet al, 2016). In Nigeria, ruminant production faces difficulty with the availability of feed during the dry season. Crop residues, concentrate diet, and agro-industrial wastes are common alternate feed sources used to overcome these issues. However, arbitrary feeding of concentrate food as a supplement to ruminants kept in confinement by smallholder farmers without careful consideration of the proportions, cost, and nutritional benefits could result in poor health status and performance as well as high production costs. Concentrate diets provide the necessary bulk for normal rumen function and the conversion of fibers into valuable volatile fatty acid components, which serves as a source of energy for both the host ruminants and rumen bacteria (Nathani et al., 2015). Based on their rumen's physiology, ruminant animals have a special feeding status. Therefore, feeding ruminant animals appropriately to suit their nutritional needs is essential for their proper nutrition. Mohammed and Chaury (2008) suggested that volatile fatty acids, a byproduct of rumen fermentation, are crucial nutrients for animal body development and rumen microbial growth.Similar to this, the rumen's patterns of fermentation in relation to the microbiota's activities serve as credible indicators of the effectiveness or ineffectiveness of dietary nutrients for the maintenance or development of the "host’s body's systems. Complete rations in ruminant feeding systems stabilize ruminal fermentation, reduce fermentation losses, and improve ammonia use efficiency (Konka et al. (2016). As a result of Nigerian smallholder farmers' arbitrary feeding of small ruminants, a complete ration, rumen fermentation and nutrient digestibility are required to determine the health status and nutrient utilization animals raised under intensive husbandry systems. Therefore, a study was conducted to determine the effects of feeding varying levels of concentrate diet on nutrient digestibility and rumen fermentation parameters of West African dwarf goats.
2. Materials and Methods
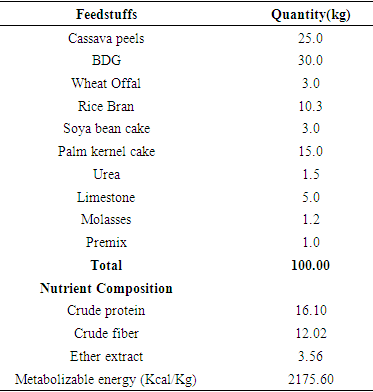
- Location of the StudyThe research was carried out at the Sheep and Goat Unit of the Department of Animal Science Teaching and Research farm, Delta State University, Asaba Campus. Asaba is located at 06°C 14’N, longitude 06° 49’E with mean temperature of 28±6°C. It has a mean annual rainfall of 1500– 1849mm with relative humidity of 77.2 – 80% (Asaba Meteorological Station, 2010).Experimental ProcedureA total of forty-five (45) West African Dwarf goats of about 4-5 months, having an initial weight between 4.70kg to 5.00 kg were obtained from goat producers within Asaba and environs for 112 days experiment. Goats were housed semi intensively in well-ventilated wooden cages in the pens. Before the arrival of the goats, the pen was cleaned, washed and disinfected with strong disinfectants solution two weeks prior to arrival. They were dewormed and vaccinated against pestes des petits ruminates (PPR). Treatment against ecto-parasites and endo parasites was done with the use of ivermectin injection. Multivitamin was also administered to boost appetite. A 7 days acclimatization period was allowed for the goats before data collection commenced. The goats were randomly assigned to 5 treatments with 3 replicates, consisting of 3 animals each per replicate.The goats were randomly assigned to five treatments with three replicates in a Complete Randomize Design (CRD). Concentrate ration was supplied on the basis of bodyweight.T1 was allotted 3.2% of their bodyweight, T2 was allotted 3.4% bodyweight, T3 3.4% bodyweight, T4 3.8% bodyweight and T5 were fed 4.0% of their bodyweight. Goats were fed basal diet of Panicum maximum.Experimental DietComposition of the experimental diet includes: cassava peel meal (CPM), brewers dried grain (BDG), rice bran, wheat offal, palm kernel cake (PKC), soya bean cake, urea, limestone, premix and molasses.
|
3. Data Collection
- Nutrient DigestibilityFollowing the feeding trial, goats from each treatment group were transferred to individual metabolism cages so that separate collections of urine and feces could be made in order to assess the goats' digestibility. The goats were kept on their pre-treatment diets and were given free access to water. Prior to the 7-day collection of urine and feces samples and the measuring of the amount of offered feed and residual feed, they were given a 7-day adjustment period. Daily feed and fecal sample collected were oven dried at 100°C for 5hours, cooled, weighed and bulked. The urinary output was collected in sample bottles with plastic cover containing 20% dilute tetraoxosulphate (VI) acid and then stored at -20°C for subsequent analysis. The fecal samples were chemically analyzed using A.O.A.C procedure (2005).Collection of rumen liquor samples and analysisAt 4 hours after feeding, 100 ml of rumen fluid samples were taken from each goat's rumen using stomach tubes and placed in sterile sample vials as reported by Babayemi and Bamikole (2006). A pH meter (3150 model, Jenway, UK) was used to rapidly detect the rumen's pH. For the purpose of separating the liquid and solid fractions without using any wash, the rumen fluid was strained through four layers of sterile cheesecloth. After then, the liquid fraction was split into two parts. The initial portion of the strained rumen fluids was acidified with 0.2 mol/L HCL solution and retained in labeled sample vials stored at -20°C until needed for the measurement of volatile fatty acids and rumen ammonia nitrogen. The second portion of the rumen fluid was preserved in a 10% formalin solution (1:9 v/v, rumen fluid: 10% formalin) and immediately placed in ice before being held at - 20°C pending additional microbial population analysis. Rumen ammonia nitrogen (NH3-N) concentration was determined by the method described by Lanyasunya et al. (2008). Total volatile fatty acids distillate concentration was determined by titration of sample with 0.1N NaOH solution expressed as volatile fatty acid content. Microbial population in the rumen fluids was by total direct count of bacteria, protozoan and fungal zoospores as described by Galyean (1989) and Dehority (2003).Statistical AnalysisAll data collected was analyzed using one-way analysis of variance (ANOVA) in a completely randomized design and significant means were separated using Duncan’s Multiple Range Test (SPSS, 2016).
4. Results and Discussion
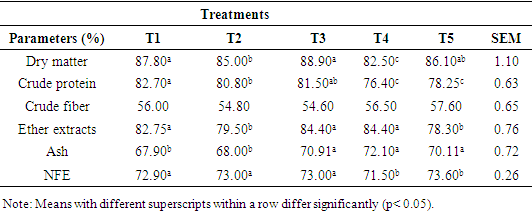
- Nutrient digestibility of WAD goats fed varying levels of concentrate dietThe apparent nutrient digestibility of West African Dwarf goats fed complete ration is presented in Table 2. The digestibility of dry matter, crude protein, ether extract, ash and NFE were influenced (p<0.05) by treatment diets. However, crude fiber digestibility was not influenced (P>0.05) by the varying the level of ration.
|
|
|
5. Discussion
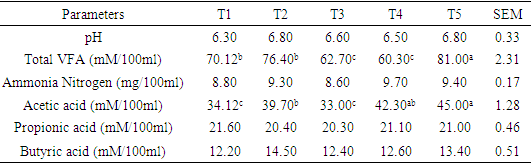
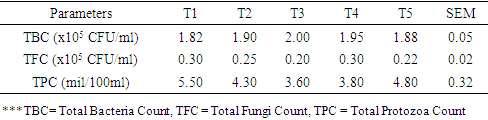
- Apparent nutrient digestibility of WAD goats fed varying levels of concentrate diet The increased crude protein content and higher consumption of the animals fed the corresponding diets may have contributed to the high dry matter digestibility found in this study. This might be accounted for by the fact that high-protein meals encourage a thriving microbial community and encourage rumen fermentation (McDonald et al., 2002). The test diets' higher crude protein digestibility coefficients showed that the animals fed those diets did a better job of using the dietary protein.The findings of this study's analysis of crude fiber digestibility demonstrated a favorable correlation between the crude protein content of diets and both crude protein digestibility and crude fiber digestibility. Since crude protein and crude fiber are parts of dry matter, any element that influences the dry matter of a feed will also have an impact on those parts of the feed as well (Ahamefule, 2005, Okpara, 2020). The higher crude fiber digestibility coefficient of the diet may indicate an increase in the activities of fibrolytic bacteria in the rumen, most likely as a result of the availability of essential nutrients, particularly protein, energy, vitamins, and minerals, which are evident in the test ingredient to promote microbial growth and multiplication. The coefficients found in this study are consistent with those reported by Ajayi et al. (2005); the present values, however, are greater than the range of values (31.96-54.43%) given by Omojola (1997). Rumen fermentation characteristics of WAD goats fed varying levels of concentrate dietThe result revealed that the ruminal pH was not significantly influenced by varying the level of diets fed to WAD goats. The pH of 6.30 to 6.80 observed in this study falls within the range of 6.00 – 7.20 pH reported by Jallow and Hsia (2011) for suitable growth and activities of microbes. However, these values are slightly higher than 6.5-6.7 recommended by Van soest et al. (1994) for a normal physiological activity in the rumen. Maria et al. (2020) reported a range of 6.0-6.9 pH for optimum growth of rumen bacteria. Additionally, according to Depeters and Bath (1996), the pH range of 6.20 to 6.80 represents the typical microbial environment and the activity of cellulolytic bacteria. According to Nagaraja (2012), the most significant ruminal factor influencing the microbial density and their activity is most likely the pH of the ruminal content. The concentration of total VFAs was found to be substantially (p<0.05) influenced by the experimental diets. The greatest concentration of VFAs was found to be 78.30 mM/100ml at T5 (4.0% bodyweight), while the lowest concentration was found to be 55.40 mM/100ml at T1 (3.2% bodyweight). The main byproduct of the microbial breakdown of fiber in the rumen is volatile fatty acid. Rumen microorganisms break down the skeletons of amino acids to create volatile fatty acids (Hassanat and Benchaar, 2013). The anaerobic microbial fermentation of complex carbohydrates in the large intestine and fore stomach produces volatile fatty acids (acetate, propionate, and butyrate) (Aluwong et al. 2010). They give more than 70% of the energy needed for ruminants (Van Soest et al., 1994). As such, a decrease in their output would be detrimental to the animals' nutritional status. More so, they operate as the foundation chains for the production of milk; acetate is a crucial element in the development of milk fat, while propionate is used to produce glucose which is required to produce lactose (Aluwong et al. 2010). The type, quantity of plant materials, and the pH of the rumen, are major factors affecting the production of VFAs. High roughage diets increases the quantity of acetate, whereas high water-soluble carbohydrate diets or concentrate based diets cause an increase in propionate (Annison et al., 2002). This was evident in this study as acetic acid values were higher across the treatments than propionic acid values as the diet was high in fibre.Rumen ammonia-nitrogen concentration ranged between 8.60 – 9.70 mg/100ml in treatment 3 and 4 respectively and was not significant influenced (p>0.05) by the experimental diets. The values were however within the range of 5 – 20 mg/100ml stated by Zareian et al. (2013) as appropriate for ruminal microbial activities. Ammonia nitrogren (NH3-N) is produced by the decomposition of rumen protein, and higher levels of ammonia nitrogen may be caused by better protein solubility, which improved crude protein digestibility (Beaucheminet al., 2003).Ruminal pH, NH3-N, and VFAs, are significant indicators of ruminal fermentation and the firmness of the rumen environment (Li et al, 2012).Rumen microbial population of WAD goats fed varying levels of concentrate dietNumerous facultative anaerobic bacteria communities are found in the rumen (Vohra et al., 2016). These communities play crucial roles in the fermentation and digestion of nutrients to produce energy and protein resources (Vymazal and Kropfelova, 2013), in maintaining key biological functions, and in fostering growth and performance. Additionally, these bacterial communities exist in a dynamic environment that is susceptible to a variety of influences, including dietary composition, diurnal variation, and feed additives (Guan et al., 2008; Menezes et al., 2011). More ruminal microorganisms can stabilize the ruminal microecosystem, increase production efficiency, and improve tolerance to external stress, according to studies in the past (Cani and Deizenna, 2009; McCann, 2001; Okpara, 2014). Rumen ammonia content and rumen fluid pH both depend on the type of diet, and both affect the number of bacteria that can be found there. Rumen bacteria make up the majority of the microorganisms in the rumen when compared to fungi and protozoa because they are the main catalyst for the fermentation of carbohydrates found in plant cell walls. It has been demonstrated that rumen fungus can break down cellulose and xylans, indicating that they may assist the ruminant host in breaking down plant components (Preston and Leng, 1987). According to Rezaeian et al. (2004), up to 8–12% of the microbial biomass in rumen is made up of anaerobic fungi that aggressively colonize plant cell walls. Caecomyces cummunis, Piromyces cummunis, and Neocallismastix frontalis are said to actively participate in the digestion of fiber in ruminants (Dey et al., 2004; Lee et al., 2004). The rhizoids of their vegetative thalli can access plant resources that would otherwise be inaccessible to other rumen microorganisms by penetrating deep into plant tissues better than bacteria and protozoa. According to Nagpal et al. (2009), this infiltration causes fodder to degrade more quickly when it enters the rumen. Cellulases, hemicellulases, xylanases, avicelases, glycosidases, etc., which have been linked to rhizomycelia (Williams et al., 1994; Lee et al., 2001), are among the very active fiber-degrading enzymes secreted by these fungi. In the small intestine, protozoa can make up to 60% of the microbial biomass, while they seldom make up more than 20% of the microbial protein flux (Newbold et al., 2014). Since ruminants are typically fed fodder diets that are poor in real protein, protozoa are now understood to have a general detrimental impact on the rumen (Bird et al., 1990). Ingesting and digesting bacteria, protozoa lower the rumen's bacterial biomass, which lowers the animals' access to protein (Coleman, 1975).
6. Conclusions
- The study indicated that feeding graded levels of concentrate diet promoted nutrient intake and enhanced digestibility in West African dwarf goats. The digestibility values obtained indicated that the diets were suitable ruminant diets. The study also revealed that concentrate based-diets influenced ruminal fermentation characteristics of goats as evidenced in the total volatile fatty acids, ruminal ammonia nitrogen concentration and microbial counts.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML