-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2022; 12(3): 65-70
doi:10.5923/j.ijaf.20221203.01
Received: Sep. 14, 2022; Accepted: Sep. 26, 2022; Published: Oct. 12, 2022

Extractant Nature on the Performance of Moringa oleifera as a Natural Coagulant for Surface Water Treatment
Medeba Uzzi1, Meresa Ramrattan2
1Department of Chemistry, University of Guyana, Georgetown, Guyana
2Government Analyst Food and Drug Department, Ministry of Public Health, Georgetown, Guyana
Correspondence to: Medeba Uzzi, Department of Chemistry, University of Guyana, Georgetown, Guyana.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The unavailability of potable water in rural communities of poor countries continues to drive investigations into the use of natural coagulants for purification of surface water. The Moringa oleifera plant has been the subject of many water purification studies worldwide. This study conducted in the South American country of Guyana aims to compare the effectiveness on water clarification of four Moringa oleifera coagulants (MOC). A 2x2x3 factorial design utilizing 2 solvents (polar and non-polar) to defat seeds, 2 solvents to extract active coagulant agent (1M NaCl and distilled water) and 3 dosages of coagulant were employed. Findings revealed that turbidity removal efficiency was not affected by the nature of the defatting solvent. Greatest turbidity removal percentages were observed in samples treated with NaCl-extracted coagulants and those receiving the highest dosages of coagulants. One outlier was the ethanol-defatted seed coagulant extracted with distilled water. Water samples treated with this coagulant had a mean turbidity of 2.5 NTU; this represented an 80% reduction in turbidity and fell within the acceptable range for potable water as outlined by the World Health Organization. There seems to be potential for the use MOC to treat surface water in poor rural communities of Guyana. Future work should investigate the use of lower salt concentrations, lower dosages and longer stirring and settling times.
Keywords: Ethanol, Hexane, Defatted seed coagulant, Dosage, Salt
Cite this paper: Medeba Uzzi, Meresa Ramrattan, Extractant Nature on the Performance of Moringa oleifera as a Natural Coagulant for Surface Water Treatment, International Journal of Agriculture and Forestry, Vol. 12 No. 3, 2022, pp. 65-70. doi: 10.5923/j.ijaf.20221203.01.
Article Outline
1. Introduction
- The process of coagulation has been used for decades – since the early 20th century to purify drinking water [1]. The advantages and disadvantages of chemical coagulation are well known and documented. Chemical coagulants containing aluminium such as aluminium sulphate (alum), aluminium chloride and polyaluminium chloride (PAC) are inexpensive, readily available, and very efficient in reducing turbidity however the cost of importation reduces the cost effectiveness of their use in developing countries [2]. Chemical coagulation processes result in the production of high volumes of sludge and significant reductions in the pH of treated water due to the presence of residual aluminium [3]. Health effects include the reported links between the use of alum and PAC to the occurrences of Alzheimer’s disease and neurotoxicity respectively [3,4]. These negative effects of chemical coagulation have led researchers to investigate other means of coagulation such as bio coagulation [5], [6], [7], [8]; utilizing plant and animal materials and more recently, electrocoagulation [9], [10], [11]; one study even utilized a combination of the two processes [12]. Several plant materials, and to a lesser extent, animal waste materials, have been studied for their coagulation ability and efficacy in treating surface water and wastewater. These include red beans, maize, peanut seeds, cactus and moringa [5], [6], [7], [8]. The Moringa oleifera (Moringaceae) plant commonly called ‘moringa’, ‘tree of life’, ‘miracle tree’ [13], ‘drumstick tree’ or ‘horseradish tree’ [14], is a native plant of North-western India [15]. Moringa widely grows in many tropical regions across the globe and is the subject of a plethora of investigations on the effectiveness of natural coagulants for water treatment across countries in Asia, Africa and South America [16], [17], [18]. The studies on Moringa oleifera have investigated the impacts of varying dosages [19–26], mechanical parameters such as agitation speed [22], agitation time [22], [24], extraction time [23] and settling time [25]; and chemical properties such as pH [21] and use of salts [26]. The addition of sodium chloride has been shown to improve coagulating abilities of natural coagulants made from Moringa oleifera [26-28]. In a recent study, Moringa oleifera coagulant extracted using 1M NaCl reduced the optimal dosage of coagulant from 170 mg/L to 140 mg/L and increased turbidity removal efficiency in wastewater from 89% to 97% [28]. The resulting salinity was 2.1 mg/L which is within the acceptable range recommended by the WHO; salinities above 3.0g/L are known to adversely affect plant life. This research explores the potential of MO as an effective turbidity reducer for the preparation of potable water from surface water. The aim is to compare the effectiveness of coagulants made from the seeds of the M. Oleifera plant using two solvents to extract seed oils (ethanol and hexane); and two solvents to extract the active coagulant agent (1M sodium chloride solution and distilled water).
2. Materials & Method
- Treatments were divided into blocks where samples were treated with different coagulants and different concentrations. Jar testing was employed to administer the coagulants.
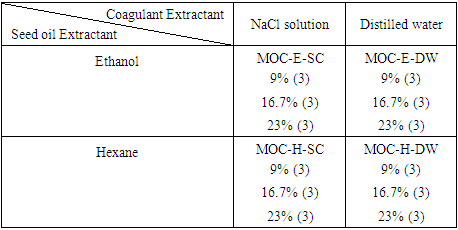
2.1. Experimental Design
- The experiment employed a 2x2x3 factorial design with three replicates per block (Table 1). The factors utilized were seed oil extractant, coagulant extractant, and dosage.
|
2.2. Preparation of Coagulant
- Dry Moringa oleifera seed pods were collected from trees on the West Bank of the Demerara River in Guyana. Seeds were removed from the pods, sun-dried, ground to a fine powder and their oils were removed by way of Soxhlet extraction for 4 hours using ethanol or hexane. The residual solid (seed cake) was collected and dried at room temperature for 24 hrs. Bioactive constituents were then extracted by mixing 25 g of the seed cake with 0.1M phosphate buffer of pH 7.5 at 6-9°C for 2 hours. This mixture was then added to either 1 L of distilled water or 1L of 1M sodium chloride solution. The resulting mixtures were stirred for 10mins (to extract the active coagulation component) followed by vacuum filtration, the filtrates were collected and refrigerated until time for use.
2.3. Sample Collection and Treatment
- Water samples were collected from the Lamaha Canal at the Guyana Water Incorporated (Shelter Belt Raw Water) in Georgetown, Guyana. The samples were collected in 1L plastic bottles. The bottles were submerged in the canal and capped underwater when full. The untreated water samples were tested for turbidity, conductivity, total dissolved solids, pH, salinity and colour. 500 mL samples of the surface water were treated with different doses of the Moringa coagulant. These doses were 50 mL, 100 mL and 150 mL which represent 9%, 16.7% and 23% v/v solutions respectively. Samples were mixed at 300 rpm for 2 minutes followed by 30 minutes mixing at 10 rpm then left to stand for 30 minutes. Water quality tests were conducted post treatment on water samples taken from the middle of the beakers. Turbidity measurements were taken using a HACH 2100 Q turbidimeter and measurements of pH, electrical conductivity, salinity and total dissolved solids were taken using a Premium Series PC60 5-in-1 multiparameter tester kit from Apera Instruments.
2.4. Analysis of Data
- The data collected was analyzed using SPSS software version 27. One-way Analyses of Variance were conducted to determine any significant differences between samples treated with different coagulants. One sample t-tests were used to compare sample means to WHO standards and the untreated water samples. All tests were carried out at a 95% level of significance (p ≥ 0.05).
3. Results & Discussion
- The results obtained were categorized according to the treatment blocks in the experimental design. They were further disaggregated into sub-blocks for discussion purposes. The findings are presented and discussed below.
3.1. Nature of Defatting Solvent on Performance of the Moringa Coagulant
- The nature of the solvent used to extract the seed oils did not impact the effectiveness of the coagulant. All the measured water quality parameters were similar for samples treated with MOC-E and MOC-H. This finding contrasts with the findings of Garcia-Fayos et al whose study showed that Moringa seed coagulants prepared using ethanol to extract seed oils via Soxhlet displayed greater turbidity removal efficiency than Moringa seed coagulants defatted with hexane or acetone. The ethanol-defatted seed coagulants required dosages that were 5 to 33 times less than the hexane-defatted and acetone-defatted seed coagulants [29]. Other studies have shown that defatted Moringa seed coagulants yield more efficient turbidity removal rates than the fatty seed coagulants [30]. Findings from other research also provide evidence for higher turbidity removal efficiency of hexane-defatted Moringa seed coagulants compared to ethanol-defatted Moringa seed coagulants [31], [32]. It is well known that non-polar solvents like hexane remove larger quantities of seed oil [33] but there seems to be inconsistencies in the reporting of turbidity removal efficiencies between hexane-defatted and ethanol-defatted seed coagulants. In the current study, the most significant difference in water quality parameter between water samples treated with MOC-E and MOC-H was observed in the pH values. Mean pH values were 6.35 and 6.08 (p = 0.051) for water samples treated with MOC-E and MOC-H respectively.
3.2. Nature of Coagulant Extractant on Performance of the Moringa Coagulant
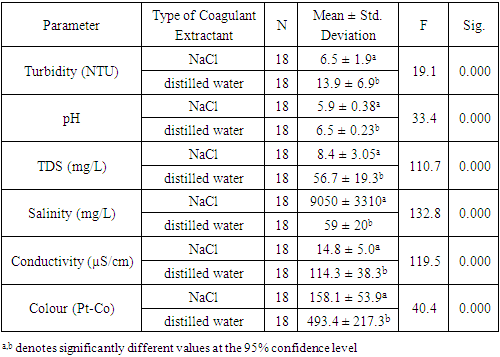
- The coagulant solutions MOC-E-SC and MOC-H-SC were more effective in turbidity removal than the coagulant solutions MOC-E-DW and MOC-H-DW. Water samples treated with MOC-E-SC and MOC-H-SC possessed lower resultant turbidity and conductivity values, less TDS and less colour. However, these samples had more acidic pH values and much higher salinities (Table 2). These findings were corroborated by Bouchareb and colleagues [28], however in their investigations the resulting salinity of treated water samples fell within the acceptable ranges for wastewater (<3.0 g/L) [29]. In the current study the resulting salinity values of samples treated with NaCl-extracted coagulants (9.0 g/L) were more than three times greater than those observed in the study by Bouchareb et al. This is due to the higher concentrations of sodium chloride solutions used in this study. Water treated with coagulants of this nature would therefore need to undergo desalination treatments before it is fit for consumption. The addition of sodium chloride improves the extraction of the active coagulant agent in Moringa (a protein). The extraction efficiency increases with an increase in sodium chloride concentration up to a certain threshold. This is known as the salting-in and salting-out phenomena [34].
|
3.3. Effect of Coagulant Dosage on Water Quality
- The addition of sodium chloride to MOC solutions greatly affected the salinity of the water samples, therefore the performance of coagulants prepared with distilled water was examined for their effectiveness. The performance of the coagulants prepared using distilled water to extract the active coagulation component also showed no dependence on the nature of the solvent used to extract the seed oils. No significant differences were found in the water quality parameters of samples treated with MOC-E and MOC-H. However, the dosage of these coagulants affected the pH and colour of the water samples. Water samples treated with the highest dosage of MOC-E-DW and MOC-H-DW possessed the least colour (colour = 315 Pt-Co and p = 0.010) and pH values that were closest to neutral (pH = 6.75 and p = 0.000).
3.4. Performance of Coagulants Prepared with Ethanol and Distilled Water
- Data was further disaggregated to focus on the effectiveness of MOC-E-DW coagulants since these materials are more readily available in a rural environment. The results showed that water samples treated with the highest dosage (23% v/v) of MOC-E-DW coagulant had the lowest turbidity (2.5 NTU); the least colour and pH values that were closest to neutral (Figure 1). It should be noted that this coagulant gave the highest turbidity removal efficiency (80%) amongst the 4 coagulants studied.
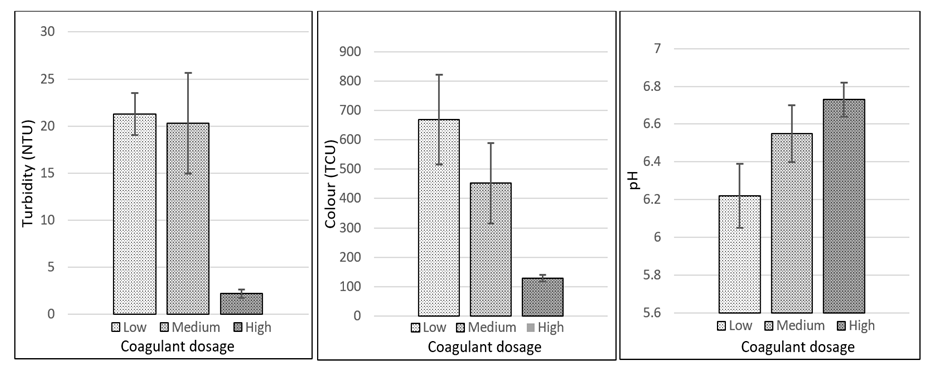 | Figure 1. Turbidity, Colour and pH values for water samples treated with various dosages of MOC-E-DW |
3.5. Performance of Coagulants Prepared with Hexane and Distilled Water
- Water samples treated with the highest dosage of MOC-H-DW coagulant possessed the greatest turbidity and TDS values; they also had pH values closest to neutral (Figure 2).
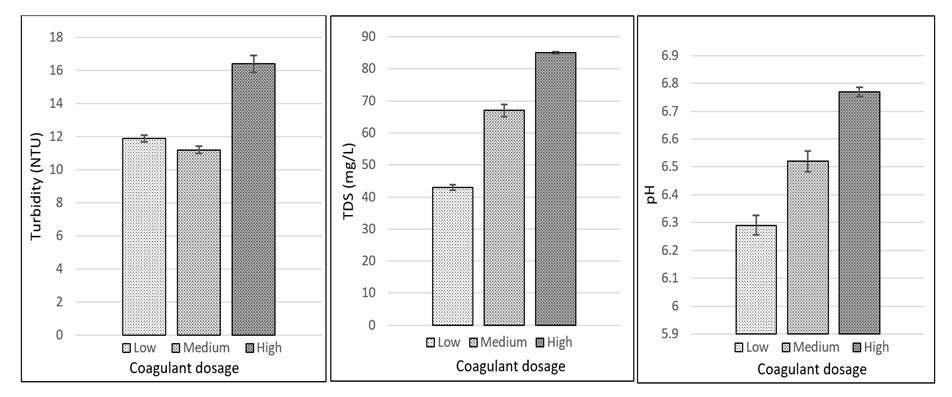 | Figure 2. Turbidity, TDS and pH values for water samples treated with various dosages of MOC-H-DW |
3.6. Comparison to WHO Standards
- The mean turbidity of water samples across all treatments was 10.2 NTU but samples treated with 23% of the MOC-E-DW coagulant had a mean turbidity of 2.22 NTU which lies within the acceptable range for potable water as recommended by the WHO. The WHO recommends turbidity values of potable water to be less than 4 NTU [35]; at this threshold turbidity can be detected visually and the water has an unappealing appearance. The acidity of water samples treated with coagulants prepared using distilled water all fell within the optimal pH range of 6.5-8.5 as recommended by the WHO, conversely samples treated with coagulants prepared using NaCl were more acidic; their pH values were below the minimum recommended threshold (mean pH = 5.92). As expected, the samples treated with the coagulants prepared using NaCl possessed salinity values which were well above the acceptable threshold for potable water of 200 ppm. The opposite was true for samples treated with coagulants prepared using distilled water (mean salinity value = 59 ppm). The samples with higher salinity values also had greater TDS and conductivity values. Nevertheless, all water samples had values of conductivity and TDS that were within the acceptable ranges as recommended by the WHO for drinking water (400 µS/cm and 600 ppm respectively). The resulting colour of the treated water samples was the single parameter that was well above the acceptable value of 15 Pt-Co. Colour values ranged between 83 and 945 Pt-Co with lowest values observed in the samples treated with 9% MOC-E-SC coagulant (mean colour = 89 Pt-Co).
3.7. Comparison to Untreated Water Samples
- The changes observed in the water quality parameters varied dependent on the type of coagulant the sample was treated with. The most desirable characteristics were observed in the samples treated with 23% MOC-E-DW. In this treatment, turbidity and colour values were lower than those of the untreated samples, pH values were less acidic and, although values of salinity, conductivity and TDS were greater than those of the untreated samples, these values fell within the acceptable ranges for safety and appearance of drinking water as recommended by the WHO. This treatment has the greatest potential as a bio coagulant for use in remote areas since the materials required for its preparation are readily available and inexpensive. The method of preparation of the coagulant is also simple and can be done using primitive equipment. The disadvantage to the use of this treatment is the high dosage that is required for effective results, given the positive results reported by other researchers regarding the use of NaCl to enhance coagulant ability, further work can be done that investigates the effectiveness of adding smaller concentrations of NaCl than were used in this study.
4. Conclusions
- The nature of the solvent used to extract the seed oils had no effect on the performance of the coagulant in reducing turbidity and other undesirable parameters from surface water. This observation improves the versatility in the preparation of the Moringa coagulant and gives scope for the use of ethanol in the commercial and domestic preparations of the coagulant. In comparison to hexane, ethanol is more readily available, less expensive and its handling poses less hazard. An additional advantage to the use of ethanol is that the resulting pH of water samples treated with the MOC-E was closer to neutral. The use of sodium chloride to extract the active coagulation component of Moringa improved performance of the coagulant resulting in lower values of turbidity, colour, conductivity, and TDS but salinity values were considerably higher. Additional costs to the water purification process would result since desalination steps would be necessary for the water to be potable. The major disadvantages to the use of the Moringa Oleifera coagulant are the high dosages required for effective water treatment and the high salinity of the resulting water samples. It is therefore recommended that further studies on the optimization of MOC include the use of ethanol for seed oil extraction and lower concentrations of sodium chloride for the extraction of the active coagulant.
ACKNOWLEDGEMENTS
- The authors thank the Centre for the Study of Biological Diversity - University of Guyana for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML