-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2022; 12(1): 1-8
doi:10.5923/j.ijaf.20221201.01
Received: Nov. 16, 2021; Accepted: Dec. 13, 2021; Published: Jan. 26, 2022

Supplemental Vitamin E and Selenium in Laying Hen Diets Enhanced Sera and Egg Cholesterol Profile
Jemiseye F. O.1, Adedeji B. S.1, Mosuro A. O.2, Olumide M. D.3, Adeyemi O. D.1, Adebiyi F. G.1, Olufeko S. O.1, Odusagun T. O.1, Adeeko A. M.1, O. A. Ogunwole1
1Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Ibadan, Nigeria
2Human Nutrition and Dietetics Department, Faculty of Public Health, Lead City University, Ibadan, Nigeria
3Department of Agriculture and Industrial Technology, Babcock University, Ilishan-Remo, Ogun State, Nigeria
Correspondence to: O. A. Ogunwole, Agricultural Biochemistry & Nutrition Unit, Department of Animal Science, University of Ibadan, Ibadan, Nigeria.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A 10-week trial was undertaken to assess the effect of dietary supplementation of vitamin E (VE) and selenium (Se) on serum and egg lipid profile of laying hens. In a completely randomized design, Bovans Brown hens (n=192) aged 52 weeks were allotted in triplicates to T1: 0mg/kg SE or VE; T2: 0.5 mg/kg-SE; T3: 1.0 mg/kg-SE; T4: 1.5 mg/kg-SE; T5: 20 mg/kg-VE and T6: 40 mg/kg-VE). Another set of hens (n=192) were similarly allocated randomly to three levels of SE (0.5, 1.0 and 1.5 mg/kg) and two levels of VE (20 and 40 mg/kg) in a 2 x 3 factorial arrangement; D1: 0.5mg/kg-SE+20 mg/kg-VE, D2: 0.5mg/kg-SE+40mg/kg-VE, D3: 1.0mg/kg-SE+20mg/kg-VE, D4: 1.0mg/kg-SE+40mg/kg-VE, D5: 1.5mg/kg-SE+20mg/kg-VE, D6: 1.5mg/kg-SE+40mg/kg-VE). Blood (3mL) and eggs were harvested at week-60 and analyzed for cholesterol profile. Serum total cholesterol (mg/dL) 59.39 in hens on T6 was similar to 98.67 in T4 and 110.54 (T1). The VLDL significantly reduced (p<0.05) from 17.30 (T2) to 7.37 (T6). Total egg cholesterol (mg/dL) of hens on T2 was similar to those from other treatments but higher than 406.95 in T3. Egg LDL in hens on T1 and T3 were lower than 258.20 in T2 but similar to those from other diets. The LDL was lowered from 60.26mg/dL (20mg/kg) to 31.02mg/dL in those on 40mg/kg VE. Sera HDL of hens on 1.0mg/kg Se was higher than 30.66 and 29.22mg/dL in hens on 0.5 and 1.5mg/kg Se, respectively. Effect of interaction of VE and Se was only significant on TC and HDL. The hens on D1 and D6 had lowered serum total cholesterol while D2 increased HDL. Also, VE, Se or their interaction had no influence on all egg lipid parameters monitored in the second study. In conclusion, Supplemental VE improved serum cholesterol, the dietary Se lowered egg TC and LDL while combinations VE and Se enhanced the serum TC and HDL, respectively.
Keywords: Bovan Nera, Vitamin E, Selenium, Cholesterol, Lipid profiles, Supplemental nutrients
Cite this paper: Jemiseye F. O., Adedeji B. S., Mosuro A. O., Olumide M. D., Adeyemi O. D., Adebiyi F. G., Olufeko S. O., Odusagun T. O., Adeeko A. M., O. A. Ogunwole, Supplemental Vitamin E and Selenium in Laying Hen Diets Enhanced Sera and Egg Cholesterol Profile, International Journal of Agriculture and Forestry, Vol. 12 No. 1, 2022, pp. 1-8. doi: 10.5923/j.ijaf.20221201.01.
1. Introduction
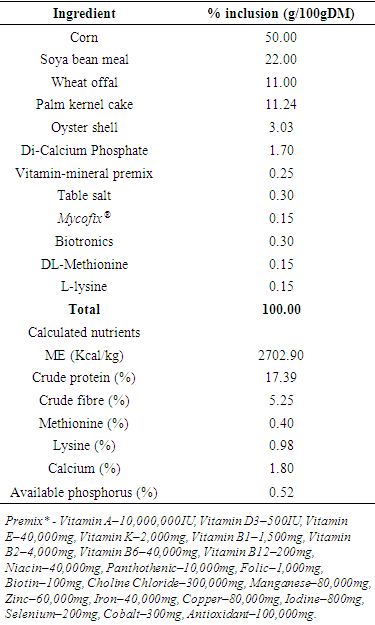
- Cholesterol is a biomolecule asynthesized in vivo by all animals and is essential in the maintenance of membrane structural integrity and fluidity [1]. [2] surmised that cholesterol along with phospholipids, serves as insulators and facilitate the speed of transmission of electrical impulses along nerve tissues. Phospholipids plays a critical role in the transport of molecules across membranes, storage and metabolism of fatty acids and as activators in blood clotting process. Triglycerides constitute the highest (about 65%) proportion of the lipids of hen. Other components like phospholipids, cholesterol and free fatty acids constitutes 29, 5% and less than 1%, respectively on dry matter basis [3]. This composition may vary depending on the age, size of the bird and nutrition. According to [3] eggs could be successfully enriched with desired constituents through dietary interventions to reduce the innate undesirable low density lipoprotein. Also, fortification of hens’ diets with additional nutrients may allow increased deposition of desired nutrients in the eggs of the hens and thus provide extra nourishment for the consumers in the form of customized or functional eggs [4].Cholesterol is an essential component of blood and is transported in association with proteins in the blood The naming or classification was therefore dependent on the type and weight of the accompanying protein [5] [6] [7] [8]. Conventionally, blood serves as a transport medium in form of lipoproteins through which cholesterol reach the target cells and organs [9]. An egg contains about 220 mg of cholesterol [10]. The cholesterol content of the egg yolk may be affected by a number of factors such as the age of the hen, genotype, rearing system and diet, and can be lowered by environmental and nutritional manipulations [11]. Vitamin E and selenium are both required nutrients by the animals. Both constitute essential nutrients needed to maintain the laying bird metabolic activity for high laying performance, reduced mortality and disease control [12]. Also, vitamin E and selenium work synergistically to improve the laying hens’ ability to resist stress, prolonged existence and sustain health with the assistance of their antioxidant properties [13].In studies with rats, supplementation with various dietary levels of vitamin E decreased cholesterol levels in the serum and liver [14]. [15] reported no effect of vitamin E on liver serum cholesterol levels. In sheep, provision of varying levels of vitamin E had no influence on plasma cholesterol [16]. [17] reported that higher dietary vitamin E led to decreased serum cholesterol concentrations of Japanese quails. However, poultry cannot synthesise vitamin E, therefore it must be given through diets [18]. Reports on effects of vitamin E and selenium on blood lipid profile of poultry are, thus, not only scanty but also grossly inconsistent. Hence, the need for this study in laying chicken. This study was therefore aimed at assessing the sole and combined effects of dietary supplement of vitamin E and selenium on blood and egg lipid profile of chickens.Experimental SiteThe experiment was carried out at the Poultry Unit of the Teaching and Research Farm University of Ibadan, Ibadan, Nigeria. The study area lies between longitude 7°27.05 north and 3°53.74 of the Greenwich Meridian east at an altitude 200m above sea level. Average temperature and relative humidity of the location is between 23-42°C and 60-80%, respectively. Laboratory procedures were conducted at the Agricultural biochemistry and Nutrition laboratory of the Department of Animal Science, University of Ibadan, Nigeria. Experimental Hens, Diets and ManagementThe details of the Basal diet composition have been published [19] and are presented in Table 1. The basal diet containing 1702 kcal/kg ME and 17% crude protein was supplemented with selenium and vitamin E as follows: T1-Basal diet, T2-Basal diet with 0.5 mg selenium/kg, T3- Basal diet with 1.0 mg selenium/kg, T4- Basal diet with 1.5 mg selenium/kg, T5-Basal diet with 20 mg vitamin E/kg, T6-Basal diet with 40 mg vitamin E/kg. Bovans Brown pullets (n=192) aged 52 weeks with good records of medication, vaccination schedules and productive performance were used for the experiment. The birds were randomly allotted to the six respective experimental diets each in triplicate.
|
2. Results
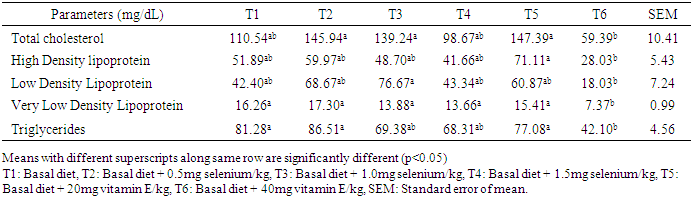
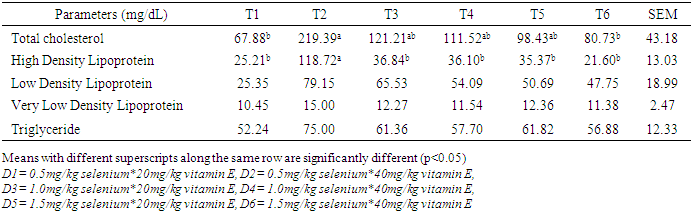
- Effect of supplemental levels of selenium and vitamin E on serum lipid profile of laying hensThe serum lipid profile of laying chickens fed diets supplemented with varying inclusion levels of selenium and vitamin E is shown in Table 2. Sera total cholesterol (mg/dL) in hens on T6 (59.39) were similar to T4 (98.67) and T1 (110.54) but significantly lower (p<0.05) to T5 (147.39), T2 (145.94) and T3 (139.24). Sera HDL in hens on T5 (71.11) was higher (p<0.05) than in hens in T6 (28.03) but similar (p>0.05) to those on other treatments. The LDL in serum of hens on T3 was similar (p>0.05) to those on T1, T2, T4, T5 but higher than (p<0.05) in T6. Sera VLDL reduced from 17.30 in hens on T2 to 7.37 in those on T6 while higher TG in T1, T2 and T5 were not different from serum TG in hens on T3 and T4 but differed significantly (p<0.05) from those on T6.
|
|
|
|
|
|
3. Discussion
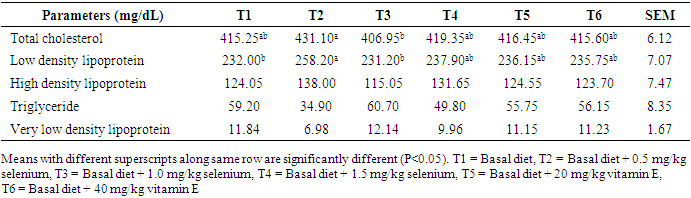
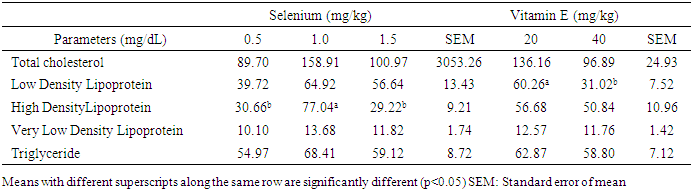
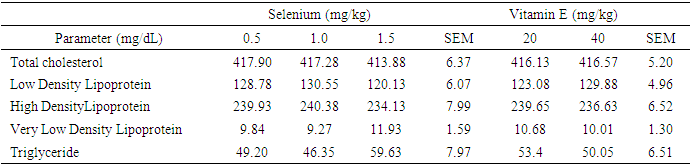
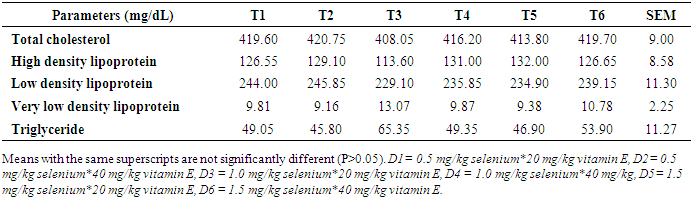
- Effects of levels of supplemental selenium and vitamin E on serum and egg yolk lipid profiles of laying hensSupplemental vitamin E and selenium enhanced the deposition of lipids in the serum. Supplemental vitamin E at 40mg/kg lowered total cholesterol, LDL, VLDL and TG. This showed that this supplemental level was adequate for the lowering of the blood cholesterol. Also, this reduction indicated the capabilities of vitamin E in the termination of damaging chain reaction caused by toxic oxygen [25]. Previous research [26] [27] had reported lowered levels of total cholesterol with increasing levels of supplemental vitamin E in diets of laying pullets. [28] [29] noted that cholesterol concentration decreased with increasing dietary antioxidant with concomitant reduction observed with increasing levels of vitamin E and selenium. It is noteworthy that cholesterol deposition in the sera of the laying hens in this study were below the normal established range (300 mg/dL) for a safe animal product consumption by the humans [30] thus emphasizing the safety of the chickens which could also mitigate cholesterol levels in the serum.Though, [31] observed that cholesterol content of poultry meat was higher than in beef and pork, the observed cholesterol levels in this study were still within the safe limit for human consumption. The HDL level in hens fed T5 increased compared to other cholesterol fraction monitored. This may be an indication that supplemental vitamin E at 20 mg/kg was adequate for healthy lipid profile. The HDL has been reported to support healthy heart functioning. This result was supported by earlier report [32] that higher serum HDL was observed at high inclusion level of antioxidant (turmeric, 0.75%) in broiler chicken diets. Also, supplemental vitamin E at 40 mg/kg reduced LDL. The LDL has been reported to be important in both the initiation and progression of plaque or increased risk for plaque rupture [33]. This suggests that vitamin E at 40mg/kg could be enough to prevent the formation/buildup of plague in the arteries. Effect of dietary vitamin E and selenium supplement on egg lipid indices, however, took a different dimension as 1.0mg/kg sufficiently lowered TC and LDL in the eggs while their effects were not obvious on other monitored egg lipid indices. This observation indicated that supplementations of the vitamins at these levels were not sufficient to effect obvious change in the lipid profile of the eggs. The cholesterol content of eggs from hens fed supplemental 1.5 mg/kg selenium, 20 mg/kg vitamin E and 40 mg/kg vitamin E were similar to the non-supplemented group. Although, inclusion of selenium at 1.0 mg/kg (T3) reduced total cholesterol in the egg yolk, while inclusion of 0.5 mg/kg selenium increased total cholesterol level. [34] reported no influence of supplemented selenium on egg yolk cholesterol. However, [35] observed reduced egg yolk cholesterol when diets were supplemented with alpha-tocopherol. [36] showed that supplemental lycopene and vitamin E separately or in combination decreased yolk total cholesterol. [37] observed that diet supplementation with 3% powdered garlic was not effective in lowering yolk cholesterol of laying chickens. Low density lipoprotein of eggs obtained from 0.5 mg/kg selenium supplemented group (T2), were not different from those supplemented with selenium at 1.5 mg/kg and vitamin E at 20 and 40 mg/kg (T5 and T6). This implies that dietary supplementation of either selenium or vitamin E produced similar impact on egg low density lipoprotein. The increased low density lipoprotein level observed in T2 (258.20 mg/dL) however may be attributed to the elevated cholesterol observed in the eggs although further supplementation up to 1.0 mg/kg selenium lowered the low density lipoprotein content of the eggs (T3). Earlier reports [38] [39] [40] postulated that antioxidants ingestion and minimal free radical exposure would reduce low density lipoprotein contributions to atherosclerosis, observation from this study was contrary. [41] observed reduced LDL when diet was supplemented with vitamin E. Increased HDL has been attributed to better health condition [42], hygienic condition of the egg [43] and freedom from contaminants which could lead to increased peroxidation and deterioration [44]. This study showed that supplemental vitamin E and selenium had no influence on high density lipoprotein composition of eggs. [45] highlighted the importance of fat content of diet on triglyceride content of the egg. This implies that fat content of the diet was not affected by dietary supplements and as such was not reflected on the triglyceride content of the eggs. The insignificant levels of very low density lipoprotein in the eggs were attributed to the insignificant levels of triglyceride as higher triglyceride has been linked to increase VLDL levels in egg yolk [46] [47]. Except TG and VLDL, lipid values in the sera were relatively lower than those in the eggs thus suggestive that the egg is an important tissue for the deposition of fat in the hen.Study 2Effect of supplemental selenium and vitamin E on blood sera and egg yolk lipid profiles Other monitored indices of lipid in this study aside from HDL and VLDL, were not influenced by dietary supplement of Vitamin E and selenium. The explanation for this may not be very clear. However, supplemental selenium improved HDL content of the serum similar to observation from study one, 40mg/kg of vitamin E successfully lowered TC. This suggests that either selenium or vitamin E has the potential to modify lipid content of the blood when included as supplement in the diets of hens. Contrary to observation from this study, dietary supplementation of 0.2% TRP (turmeric) to laying hens resulted in lower serum triglyceride and total cholesterol concentrations [48] while inclusion of 0.1% or 0.5% curcumin in rat diets lowered the concentrations of cholesterol in the liver and serum [49]. Selenium plays a crucial role in controlling the effects of thyroid hormone on fat metabolism [50]. From this study, lipid profile of the eggs was not influenced by supplemental selenium. This observation was contrary to the observation of [50] that supplemental selenium reduced yolk triglyceride and cholesterol. Similarly, [51] established that higher dietary selenium levels significantly lowered cholesterol concentration in chickens. The TC, HDL, LDL, VLDL and triglyceride content of eggs collected were not affected by supplemental vitamin E. Convesely, [41] showed reduced cholesterol, triglyceride, VLDL, LDL and increased HDL with dietary supplementation of vitamin E. Also, [52] reported improved HDL when diets of rabbits were supplemented with dietary vitamin E. Effects of interaction of dietary supplement of vitamin E and selenium on blood sera and egg yolk lipid profiles Selenium and vitamin E are inter-related, hence complete protection of living cells requires both vitamin E and selenium in the diet. The effect of these two antioxidants were more obvious on TC and HDL as 1.5mg/kg Se+40mg/kg vitamin E successfully lowered TC while 0.5mg/kg selenium+40mg/kg vitamin E improved HDL profile of the sera lipids. Though lipid TC in hens on D2 was high, the proportion of HDL however was more than half of the TC content thus suggesting that dietary supplementation with 0.5mgSe+40mgVE increased sera HDL compared favorably with other levels of antioxidant combinations. This implied that the likelihood of arterial development of plaques due to these combinations would be remote. [41] earlier reported that combination of antioxidants reduced the lipid profile of the serum. Similarly, [53] reported reduced cholesterol profile of rats fed two sources of antioxidants. Conversely, [54] reported no change in serum total cholesterol, HDL-c, LDL-c and triglyceride concentrations in broiler chickens given diets supplemented with 0.1% or 0.2% TRP alone or with aloe vera powder. [36] reported that combination of dietary lycopene and vitamin E supplementation, significantly reduced serum cholesterol concentrations in Japanese quails. The interaction of dietary selenium and vitamin E showed no influence on egg lipid profile therefore suggesting that a higher level of combined dietary vitamins would be required to modify the lipid profile of the egg yolk.
4. Conclusions and Recommendations
- Supplemental selenium at 1.0 mg/kg and vitamin E at 20 mg/kg levels in the diet enhanced serum HDL composition while combined dietary supplement of 0.5 mg/kg selenium and 40 mg/kg vitamin Eincreased the sera HDL. Egg yolkcholesterol and LDL werereduced by seleniumsupplementation at 1.0mg/kg while interaction of selenium and vitamin E had no influence on lipid profile of eggyolk. Further studies should be undertaken on the effect of supplemental vitamin E and selenium on nutrient deposition, shelf life and stability of egg.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML