-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2021; 11(3): 52-60
doi:10.5923/j.ijaf.20211103.02
Received: Dec. 3, 2021; Accepted: Dec. 29, 2021; Published: Dec. 30, 2021

The Efficiency of (Bentonite) Organoclays in Removing Oils from Fast-food Effluents: A Systematic Review
Allande Johnson-Hackett
Univerisrty of Guyana, Faculty of Earth and Environmental Sciences, Georgetown, Guyana
Correspondence to: Allande Johnson-Hackett, Univerisrty of Guyana, Faculty of Earth and Environmental Sciences, Georgetown, Guyana.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The effective removal of oil from water (oily-water) was achieved using the centuries’ old method of activated carbon. In recent times, however, other methods using dispersants, absorbents, solidifiers, booms and skimmers were developed. These current methods of oil adsorption have varying levels of efficiency or are limited to specific oils. Recently research has highlighted the use of organoclays, a natural compound that has been proven to be both effective and efficient in oil removal from wastewaters. Organoclays have be noted to remove seven (7) times or more than its dry weight in oil making it more efficient than activated carbon. In this review, the researcher focused mainly on studies in which organoclays have been most effective. It is visually noted that the drains near fast food outlets have been observed to always contain oil contaminated wastewater, therefore these were the target zones in this review. The aim was to assess those approaches to ascertain the most effective design(s) for efficient oil removal from wastewater being released from fast food outlets.
Keywords: Organoclays, Oil removal, Wastewater, Removing fast-foods oil residues from water
Cite this paper: Allande Johnson-Hackett, The Efficiency of (Bentonite) Organoclays in Removing Oils from Fast-food Effluents: A Systematic Review, International Journal of Agriculture and Forestry, Vol. 11 No. 3, 2021, pp. 52-60. doi: 10.5923/j.ijaf.20211103.02.
Article Outline
1. Introduction
- Organoclays are classified as organically manufactured compounds made by modifying bentonite (natural clay) with quaternary amines (ammonium salt) [1]. The quaternary amines are surfactants, which have a water loving (hydrophilic) and oil loving (lipophilic) end [2]. Organically modified bentonite clays, or organoclays, have been in use for more than four (4) decades, mainly as commercial thickeners and flow-control additives in solvent-based systems (2003) [3]. The diagram below illustrates the development of the organoclay compound. It shows the group of smectite clay minerals composed of two silicate tetrahedral sheets with a central octahedral sheet, joined by common oxygen atoms to the leaves. The leaves are continuous in the directions of crystallographic axes (imaginary lines within the crystal lattice). The clay then treated with quaternary salts develops the ability to swell by adsorption of organic molecules, resulting in the organoclay.
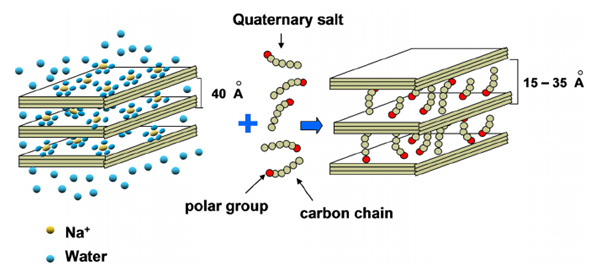 | Figure 1. Diagram showing the development of an organoclay compound (Source: (Cavalcanti Abreu, Carvalho & Motta, 2012) [4]) |
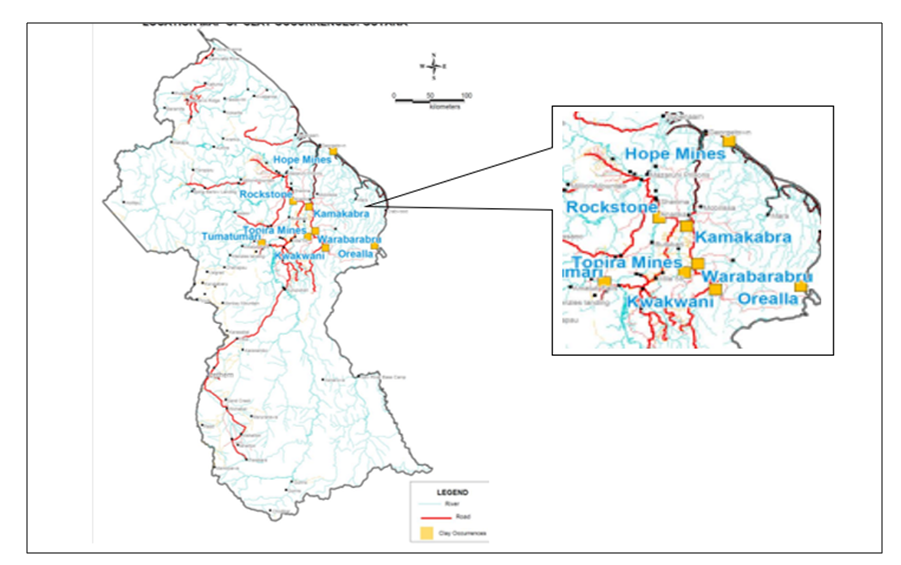 | Figure 2. Map showing clay deposits in Guyana provided by Geology and Mines Commission. (Source: Vieira (2014) [8]) |
|
 | Figure 3. FOG Residue Clogging Local Drains Connected to Sewers (Source: Unknown (2021)) [24]) |
|
2. Methodology
- The research strategy applied in this review on organoclays consisted of literature searches in Google scholarly, Z-books, JSTOR, SCOPUS and EBSCO archives through the University of Guyana student access link. These platforms provided access to thousands of scholarly pieces of literature from 2000 to 2020. However, the use of organoclay studies were limited in number, thus, a more thematic approach was adopted. This review provides highlights of research studies that have been published on the efficiency and effectiveness of organoclays in removing oil from wastewater using a variety of methods and use of organoclays in varying forms were selected. The studies reviewed were all linked to Alther’s theory, which stemmed from Jordon’s work in the 1940s cited by Chaiko (2006) in their article on Preparation of Organoclays [26]. With this focus, the review was narrowed to five (5) methods using organoclay where they have been deemed efficient and effective to assess and make inferences regarding the best possible method(s) for removing oils from waterbodies polluted mainly from fast food outlets.No studies on the use of organoclays to remove oil from wastewaters were found in Guyana, thus all studies examined were from international sources. However, since there seemed to be a commonality in oil residue in drains where fast food industries are present, regardless of country, these methods would find universal application. These database searches were neither temporal nor chronological. Articles that matched the theme were pre-screened based on their abstracts. In some cases, introductions, methods and conclusions were reviewed in order to guide the final selection for this systematic review. It must be noted however, that studies retrieved on organoclays were based mainly from chemists’ standpoints while those on restaurant pollution of oils were viewed from a biologist’s viewpoint. Therefore, connections were made to ensure the inclusivity from all angles. The articles selected were then reviewed and the most significant data relating to the theme were extracted for use in this study. Emphasis was placed on the outcomes of the studies and the variability in the methods and their successes or failures. The data was then analyzed and subsequently used to present this review using a thematic approach before an overall conclusion was drawn and recommendations made for future research.
3. Evaluating Methods to Remove Oil Using Organoclays
- 1) Powdered bentonite organoclayPowdered bentonite organoclay has been assessed in several studies to determine its efficiency in oil removal. These studies were based on the Freundlich’s model, which is premised on dispersing powdered bentonite organoclay on the surface of oil-based wastewater to observe its oil adsorption tendencies. One such study was conducted by Okiel, Mohamed & El-Kady (2011) in Egypt [27].However, these studies mainly compared the Lagergren model of 1989 that was remodeled by Alther in 1995, which all yielded the same outcome of 70-90% oil adsorption. In one study by Viraraghavan & Moazed [28] based in Estevan Saskatchewan, Canada, the method by Alther (1995), the Lagergren’s model, was compared using a different method called the Freundlich model, to determine the best efficiency model for oil removal. While the article provided evidence that organoclays were effective in oil removal, it did not provide a kinetic analysis nor did it examine the applicability of absorption isomers to oil removal by organoclays, thus the true efficiency of the organoclays were not determined. Viraraghavan & Moazed [28] used four types of oils: mineral oil, two cutting oils (Kutwells-45 and Valcool) and refinery oil with concentrations ranging from 26 mg/L to 381 mg/L, to investigate the potential of powdered bentonite organoclay on oil removal. The study compared oil removal from synthetic and actual oil in water emulsions, to evaluate the adsorption capacity. The powdered organoclay was dispersed into the wastewaters in batches using a two-phase system. The first phase lasted between 15 to 30 minutes, when rapid absorption occurred. This accounted for about 70 to 99% of the oil being removed from the waterbody during this phase. The second phase was much slower with very little oil being removed in this phase. Most of the oil adsorption occurred during the first phase with an overall equilibrium time of one hour. The report further stated that both the Lagergren’s model and the Freundlich model used showed a 95% statistical confidence level to be able to describe the absorption kinetics for the mineral oils and the two cutting oils but no kinetic analysis was done. This indicated that the absorption capacities were high for the cutting oils, but even higher in the Freundlich’s model than in the Lagergren’s model. The contrary occurred with the refinery oil which had very low adsorption values of approximately thirty-seven percent (37%). Viraraghavan & Moazed [28]) concluded that because the refinery oil was already pre-treated and were highly stable, then the significant levels of adsorption were very low and correlation non-existent. While the article provided evidence that organoclays were effective in oil removal, it did not provide a kinetic analysis nor did it examine the applicability of absorption isomers to oil removal by organoclays, thus the true efficiency of the organoclays were not determined. The bentonite organoclay was deemed efficient and the batch system applied was found to be suitable for treating wastewater in small volumes. It was also stated that organoclays would have the ability to remove up to 100% of oil from oil in water emulsions, but it is contingent upon the equilibrium time of contact. Therefore, the authors concluded that Freundlich’s model provided the best isomers in this study for absorption of oils, since the oil removal capacity was greater than that discovered by Alther’s study in 1995.Another advantage discovered in the Alther’s study was that bentonite organoclays had the ability to also remove heavy metals such as lead, copper and nickel from its solvent. Other oil removal methods did not possess this capability. However, the study did not indicate to what degree heavy metals were removed in this regard.In a follow-up study Kshash, [29 and a previous study by Watson [30], similar results were observed where it showed organoclays being 70-90% oil adsorption capacities, but it emphasized the fact that the quantity of quaternary amine that was used in preparation of organoclay had very significant effects on the removal of oil from wastewater. In this regard, it was the weight ratio of quaternary amine in the organoclay increased, the oil adsorption capacities also increased. Kshash [29] also found that the rate of flow of the substance through the organoclay being used was also important, since the faster the speed of the mixers, the thinner the oil, thus filtration occurred faster [27] [28]. The same conclusion from Ksash’s study was stated in research done by Chidi, Nnanna & Ifedi [31]]. In their study, the bentonite used was mined in Nigeria and the phenol used in the research was obtained from Guangdong, China with chemical formula C6H6O. In this research they also concluded that “by increasing the time of agitation of the modified bentonite in the phenol solution it would increase the adsorptive properties of the organoclay”. Chidi, Nnanna & Ifedi [31], study went a little further and addressed the kinetic analysis that was lacking in Viraraghavan & Moazed’s (2005) research [28]. These researchers, Chidi, Nnanna & Ifedi [31], stated that the adsorption process fit well with the pseudo second-order kinetic model equation and confirmed that weak chemical interactions controlled the rate adsorption step of phenol, which simply meant that the rate of adsorption was highly dependent on the initial concentration of phenol solution. These studies have all concluded that bentonite organoclay is effective in removing oil from water with an efficiency ratio being above 70% in every instance. Agitation of the solution through the mixture, multiple passes and the weight ratio of quaternary amine in the organoclay were all major contributing factors. The diagram in Figure 4 illustrates how the removal of contaminants from waste water would appear with their bonding structure. This study was done in Iran where low-cost Iranian bentonite was modified and used to adsorb the removal of pollutants with physiochemical properties like phenanthrene (PHE) and copper ions (Cu2+), and so on, from both simulated and real wastewaters.
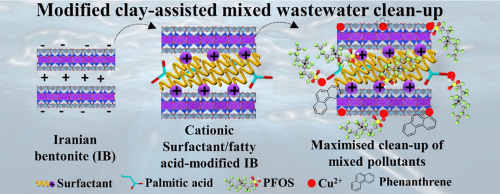 | Figure 4. Modified clay structure being mixed with waste water to show removal of pollutants (Source: (Khodabakhshloo, Biswas, Moore, Du & Naidu,2021, volume 800, ISSN 0169-1317) [32]) |
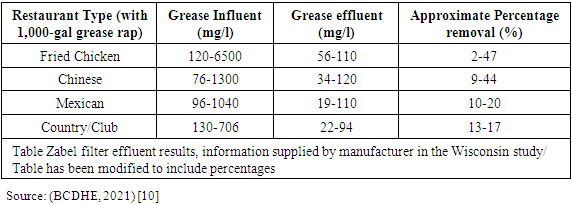
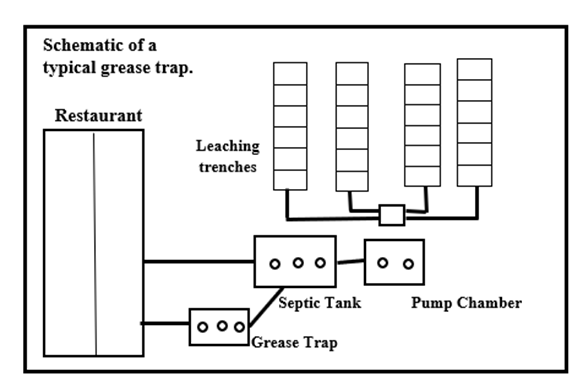 | Figure 5. Diagram of schematic view of a typical grease trap set up and its connection to sewer tanks (Source: (BCDHE, 2021) [10]) |
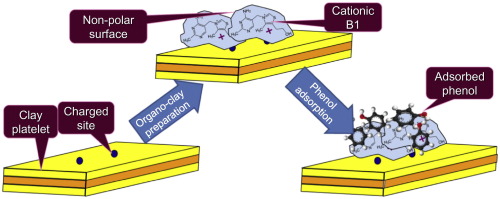 | Figure 6. Showing organoclay preparation and its non-polar and cationic charge. (Source: (Moshe & Rytwo, 2018, pg. 50-56) [41]) |
 | Figure 7. Illustration of oil and grease residues being removed with the use of organoclay and two compeitors from conventional methods. (Source: (Ecologix, 2018) [43]) |
4. Conclusions
- Of the four methods reviewed, it was observed that there was no one-size-fits-all approach for FOG waste removal. However, the effectiveness of the use of the organoclay compounds in removing or adsorbing oils from waterbodies was consistent regardless of the way it was used, its kineticist or its chain properties. What varied was its efficiency in the varying forms of adsorption and the weight ratio of quaternary amine in the organoclay and if agitation was being used were all major contributing factors. As was initially presented in the research, the short chain organoclays worked well on surface oil that was thinly spread. Therefore, applying bentonite organoclay in a powdered form on the surface of the water and skimming the amalgamated organoclay and FOG compound from the surface can be a measure implemented at the end of each working week or working day, depending on the level of waste being produced by that fast food outlet. FOG should not be thrown down the drains, however, regardless of the steps taken in commercial restaurant settings, some amounts of oil or fat will pass through the system. Thus, organoclay filters attached directly to the drainage pipes in the sinks with several rotating systems of connecting pipes would be very beneficial. This would significantly reduce levels of oil residue being released into the environment or connecting sewer systems. In all the research cited, organoclays were deemed to be more than seventy percent (70%) effective, and a series of systems would be more likely to take that percentage closer to one hundred percent (100%) for any waste being released through those systems. However, there is a need for more industrial and academic in-field work to be done on the use of organoclays to determine if its long-term exposure or use in the environment would have adverse side effects, so that organoclays can become a much more first line approach to tackling fast food FOG issues instead of conventional methods that have lower efficiency readings.
ACKNOWLEDGEMENTS
- Special thank you to my husband, parents, family members and friend, Dr Corbin, for giving me the support and views needed to complete this assignment. To my supervisors: Mr. Fredericks and Ms. Uzzi, who have worked despite the setbacks from the pandemic, I say thank you. Above all, I thank God for perseverance, which He graciously bestowed on me. The struggle was real.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML