-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2021; 11(1): 16-23
doi:10.5923/j.ijaf.20211101.03
Received: Apr. 9, 2021; Accepted: Apr. 23, 2021; Published: May 15, 2021

Evaluation of Antioxidant Traits in Fruits of Some Hot Pepper (Capsicum sp.) Genotypes under Greenhouse and Field Conditions
Rexford Ackey1, George Oduro Nkansah1, 2, Essie T. Blay1, 3, Isaac Kwadwo Asante3, 4, Kingsley Ochar1
1Department of Crop Science, University of Ghana, Legon, Ghana
2University of Ghana Forest and Horticultural Crops Research Centre, Ghana
3West Africa Centre for Crop Improvement, University of Ghana, Legon, Ghana
4Department of Plant and Environmental Biology, University of Ghana, Legon, Ghana
Correspondence to: Rexford Ackey, Department of Crop Science, University of Ghana, Legon, Ghana.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Antioxidants are beneficial for the management of some chronic diseases such as cancer, neurodegenerative, cardiovascular and diabetic diseases. Through scavenging activities on free radicals, antioxidants reduce oxidative stress in human. Plant sources of antioxidants are better in potency and health risks than the synthetics, especially in fruits and vegetables. Research has established that consumption of vegetables reduces the risk of many chronic diseases due to the presence of antioxidants. Therefore, this experiment was conducted using seventeen (17) hot pepper (Capsicum spp.) genotypes to find out their antioxidant compositions. All samples for the genotypes were extracted using methanol and ethanol solvents. Total phenolic content was determined by using Folin-Ciocalteau method on absorbance at 765 nm. Lycopene and β-carotene were determined through the modified method of Sharoba on absorbanceat 453nm, 505nm, and 663. For total flavonoid content, the modified aluminum chloride colorimetric method was used on absorbance at 510nm. Antioxidant activity was determined using 2, 2-diphenyl-1-picrylhydrazyl (DPPH) free radical scavenger method. The phenolic acids content recorded under field conditions were relatively higher in all genotypes than under greenhouse conditions. For carotenoids, whereas high lycopene content was recorded under greenhouse conditions β carotene yield was rather higher under field conditions. Total flavonoid content among the genotypes varied across the two experimental environments. DPPH free radical scavenging activity was generally high and showed a correlation with phenolic acids content across the environments. To conclude, the antioxidants studied among the genotypes were high in concentration and varied across environments.
Keywords: Genotypes, Antioxidants, Environments, Concentrations, Pepper, Evaluation
Cite this paper: Rexford Ackey, George Oduro Nkansah, Essie T. Blay, Isaac Kwadwo Asante, Kingsley Ochar, Evaluation of Antioxidant Traits in Fruits of Some Hot Pepper (Capsicum sp.) Genotypes under Greenhouse and Field Conditions, International Journal of Agriculture and Forestry, Vol. 11 No. 1, 2021, pp. 16-23. doi: 10.5923/j.ijaf.20211101.03.
Article Outline
1. Introduction
- Hot pepper (Capsicum spp.) is one of the most divergent crops cultivated globally. It is ranked the third most important vegetable besides peas and tomatoes (Ochoa-Alej and Ramirez-Malagon, 2001; Ali, 2006). It is extensively used as food in the world over (Terry and Boyhan, 2006) perhaps due to the presence of many health promoting substances. In recent times, the potency of pepper as a source of natural antioxidants has been recognised and now being exploited through research. Antioxidants are phytochemicals that delay, inhibit or take away oxidative damages to cells in the body. Thus, they prevent or reduce oxidative events within the body (Halliwell, 2007) through scavenging of free radicals from the cells (Dolas and Gotmar, 2015). Antioxidants in general contribute to solving many health related issues in humans. They prevent cardiovascular, carcinogenic and neurological diseases (Williamson et al., 2000; Morton et al., 2000) such as rheumatoid arthritis, cancers, eye, heart, alzheimer and parkinson’s diseases (http://www.betterhealth.vic.gov.au/bhcv2/bhcarticles.nsf/pages/Antioxidants). Comparatively, natural antioxidants are better than the synthetics, in that they have multiple health benefits with no adverse effects, readily acceptable by the body, and have antioxidant effects on human tissues (Reena et al., 2016). Thus, consumers’ quest for natural antioxidants and restriction on the usage of synthetic antioxidants as well as people’s awareness of its health-related issues (Vazquez et al., 2012) has pushed for greater investigations into these. Moreover, natural antioxidants are safer and have even greater antioxidant activity compared to the synthetics (Beutner et al., 2001). Natural antioxidants are obtained from secondary metabolites available in fruits and vegetables. These include compounds such as carotenoids, alkaloids and phenolics (Hollman, 2001). Among these, polyphenols and carotenoids are the two main compounds which account for the highest antioxidant properties in plants (Zhang et al., 2013). Flavonoids dominate phenolics in plant foods (Bors et al., 1996) and are estimated to be two-thirds of all dietary phenols (Scalbert, and Williamson, 2000). Natural carotenoids are beneficial in the prevention of numerous types of cancers and other diseases. Among carotenoids, lycopene is by far the most potent antioxidant known because it possesses the highest singlet oxygen quenching rate (Rao and Rao, 2007). Also, it is able to fight cancers such as prostate, urinary bladder and colon cancers. Beta-carotene is also known to reduce the dangers of stroke, heart diseases, aging, vascular and other metabolic diseases (Shankaranarayanan et al., 2018). Like other vegetables, hot pepper (Capsicum spp.) genotypes contain most of the secondary metabolites which are very essential for human health. Phenolics have been reported to be higher in hot pepper (Capsicum spp.) than all other vegetables sources (Kumar et al., 2009). A considerable intake of hot pepper (Capsicum spp.) could boost the antioxidants content and activities in man which in effect reduces the threat of carcinogenesis (Nishino et al., 2009). As a result of the rise in preference for natural antioxidants by consumers, this research was set out to establish the following in some seventeen (17) hot pepper (Capsicum spp.) genotypes:a) To determine the concentrations of antioxidants traits (phenolics and carotenoids) in the pepper genotypes. b) To compare the performance of the antioxidant (phenolics and carotenoids) traits under two different environments (greenhouse and field conditions). c) To assess the impact of locations (greenhouse and field conditions) on the antioxidants traits (phenolics and carotenoids) in the pepper genotypes.
2. Materials and Method
2.1. Experimental Site
- This experiment was conducted under greenhouse and field conditions. Crop cultivation and growth were done at the Forest and Horticultural Crops Research Centre (FOHCREC) of the University of Ghana, Kade in the Eastern Region of Ghana. Laboratory analyses of antioxidant contents of the pepper genotypes were done at the Department of Botany, College of Basic and Applied Sciences of University of Ghana Campus, Accra.
2.2. Pepper Genotypes used for the Experiment
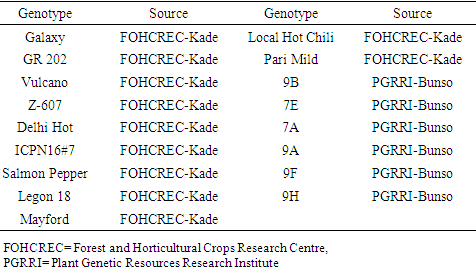
- We studied a total of Seventeen (17) genotypes obtained from Plant Genetic Resources Research Institute (PGRRI), Bunso and the Forest and Horticultural Crops Research Centre (FOHCREC), Kade both in the Eastern Region of Ghana (Table 1).
|
2.3. Experimental Design
- The experiments were laid out in randomized complete block design with three (3) replications for each genotype. Genotypes were assigned to plots randomly using the drawing lots method.
2.4. Planting Distances under Greenhouse and Field Conditions
- Under field conditions, plants were sown on ridges with the dimension of each as 82.6 m x 0.53 m and 0.7 m separating each of them. Seedlings were planted with the distance of 0.8 m × 0.59 m. For the greenhouse conditions, beds were 6m in length and 0.95m in width. A distance of 0.54m separated the beds. The planting distances were 0.6 m × 0.5 m.
2.5. Nursery and Cultural Practices
- Seeds were sown in compartmentalized seed boxes filled with rice biochar (carbonated rice husk) as the medium. Two seeds were sown per cell. Effective watering was undertaken after germination until transplanting. To check damping-off disease at the nursery, the seedlings were sprayed with appropriate fungicide at two (2) weeks intervals until transplanting. Transplanting was done four (4) weeks after sowing of seeds.
2.6. Laboratory Analysis of Fruit Quality Traits
- Laboratory analyses were performed for the following antioxidant traits of all the pepper genotypes: phenolic acids (gallic, vanillic, and rosmarinic acids), total flavonoids, lycopene and β-carotene contents as well as antioxidant activities (IC50 value). Dried and ground fruit samples of all the pepper genotypes were used for these analyses.
2.6.1. Drying of the Fruits of Pepper Genotypes
- Pepper fruits used for the chemical analyses were in the fully ripe state. Non-specified sample of ripe fruits was oven-dried at 60°C for forty-eight (48) hours as described by Ikpeme et al. (2014).
2.6.2. Preparation of Pepper Samples
- Powdered pepper samples were prepared using the approach adopted by Tsai et al. (2009) with a slight modification. The fruits were pulverized into fine powder. Ten (10) grams of the pulverized sample of each genotype was extracted with 100 ml of methanol at 25°C at 20xg for 24 hours and later filtered through Whatman No. 1 filter paper. The residue was extracted with two additional 100 ml portions of methanol as described above and combined ethanolic extracts were concentrated under reduced pressure below 40°C to obtain the crude extract. The crude extracts were re-dissolved in methanol at concentration of 20 mg/ml and stored at 4°C for further analyses.
2.6.3. Determination of Total Phenolic Content
- Total phenolic content was determined by using Folin-Ciocalteau reagent based on modified version of the method by Harborne (1989). A volume of 1.0ml from each pepper genotype’s sample was added to 1.0ml aqueous sodium carbonate solution. A 1.0ml volume of Folin-Ciocalteau reagent was added to the mixture and topped up to 10 ml. The mixture was agitated and allowed to stand for 90 minutes. The absorbance was measured at 765 nm by using UV/visible spectrophotometer (SpectraMax Plus 384, United states). The concentration of the total phenolic compounds was calculated based on standard curve of gallic acid (0.2 – 1.0 mg/ml) with the linear equation, y = 0.624x – 0.939, where R2 = 0.995. The results were expressed as mg of gallic acid equivalent (GAE/mg) per 100 ml of the extract. For the determination of the concentrations of individual phenolic compounds, the following formulae were used: (a) gallic acid (mg/100ml): y = 0.0871x – 0. 102 (b) vanillic acid (mg/100ml): y = 0.053x + 0.012 (c) rosmarinic acid (mg/100ml): y = 0.069x + 0.022.
2.6.4. Determination of Lycopene and β-Carotene Contents
- The modified method of Sharoba (2009) was followed for this determination. To determine the concentrations of lycopene and β-carotene, the absorbance of the extracts were measured at the wavelengths 453nm, 505nm, and 663 by using spectrophotometer (SpectraMax Plus 384, United states). The following formulae according to Nagata and Yamashita (1992) were used to calculate for the concentration of lycopene and β-carotene respectively; a) Lycopene (mg/100ml) = -0.0458A663 + 0.372A505- 0.0806A453 b) β-carotene (mg/100ml) = 0.216A663 – 0.304A505 + 0.452A453.
2.6.5. Determination of Total Flavonoid Content of Pepper Genotypes
- The modified aluminium chloride colorimetric method by Barros et al. (2007) was used to determine the flavonoid content. Methanolic extract of pepper fruits (0.5 ml) was mixed with distilled water at 500 µl and sodium nitrite, NaNO2 (5%, 30 µl). The mixture was allowed to stand for 5 minutes. Aluminium chloride solution, AlCl3. H2O (10%, 60 µl) was added to the mixture. The mixture was allowed to stand for 6 minutes after this. Sodium hydroxide, NaOH (1M, 200 µl) and distilled water of 110 µl were added to the mixture and made to thoroughly mixed. Absorbance was taken at 510 nm (SpectraMax Plus 384, United States). Concentration of total flavonoid content was computed based on standard curve of rutin (0.2 – 1.0 mg/ml) with the linear equation y= 0.0101x + 0. 2238 with R2 = 0.9563. The results were expressed as mg of rutin equivalent (RE/mg) 100 ml of the extract.
2.6.6. Determination of Antioxidant Activity of Pepper Genotypes Chemicals and Reagents
- The chemical DPPH was secured from Sigma Aldrich Co. (St. Louis, USA). Other chemicals used were of analytical grade.
2.6.7. 2, 2-Diphenyl-1-picryhydrazyl (DPPH) Free Radical Scavenging Activity of Methanolic Extract
- Diluted working solutions of the test extracts were prepared in methanol. The standard used was ascorbic acid. A volume of 100µl of test samples (0.6-20mg/ml) measured accurately in methanol was added to 5 µl DPPH solution. A percentage of 0.002 DPPH was made in methanol. A microliter of the DPPH solution was mixed with 1 ml of sample solution and a standard solution to be tested separately. The solution mixture was kept in the dark for 30 minutes. Afterwards, an optical density was measured at 517 nm by a spectrophotometer against 1 ml methanol as the blank in 1 ml of DPPH solution (0.002%). The optical density was recorded and percentage inhibition was calculated using the formula by (El-Agbar et al., 2008). The percentage inhibition of DPPH activity
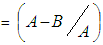 Where;A- Optical density of the blankB- Optical density of the sample
Where;A- Optical density of the blankB- Optical density of the sample2.7. Statistics and Estimation of IC50
- The development (decolorization) was plotted against sample extract concentration. A linear regression curve was established for the calculation of IC50 (µg/ml). IC50 indicates the value of the sample needed to reduce the absorbance of the DPPH radical by 50%. All phytochemical analysis was carried out in triplicate and the averages were estimated.
2.8. Analysis of Data
- All data from the experiments were analyzed by using the GenStat Computer Statistical Software (2009) and XLSTAT statistical software (2015).
3. Results and Discussion
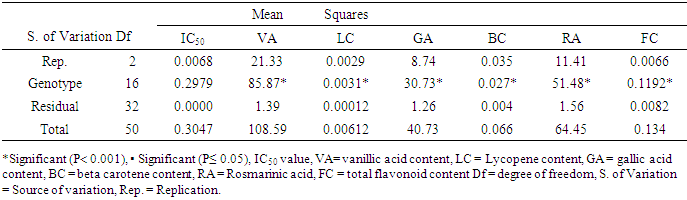
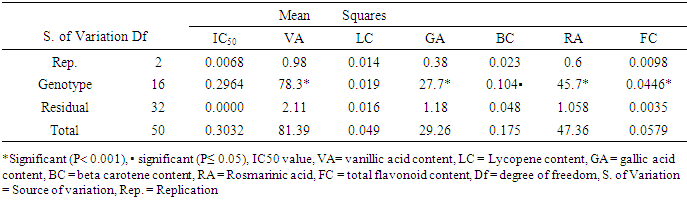
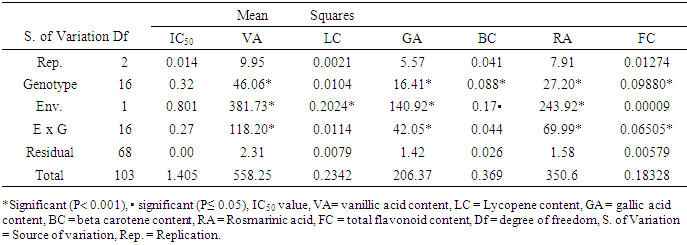
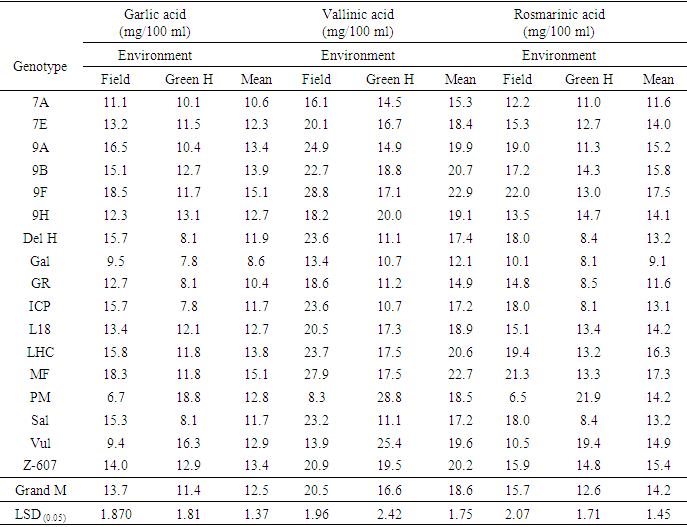
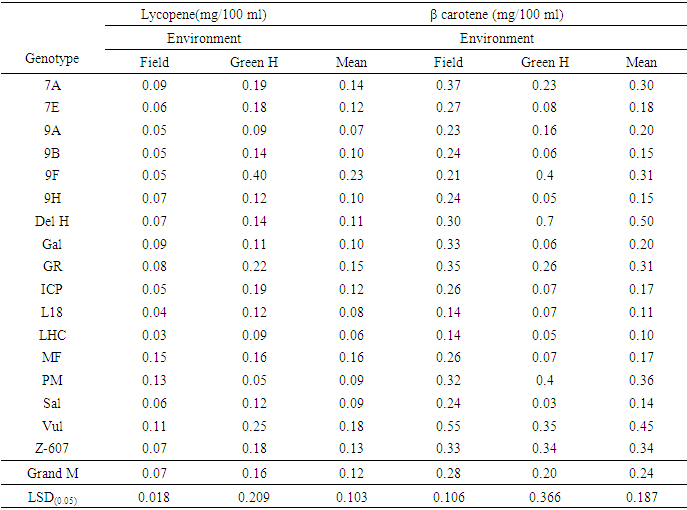
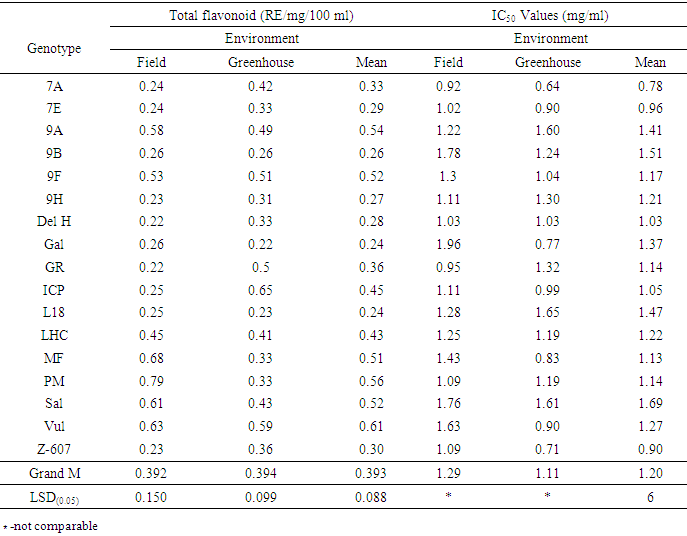
- Analysis of variance (ANOVA) performed for all the antioxidant parameters revealed significant difference (P< 0.001 and P≤ 0.05) across genotypes, environments, and genotype x environment. However, IC50 under both experimental conditions and lycopene under greenhouse conditions were not significant (Tables 2, 3 and 4).
|
|
|
|
|
|
4. Conclusions
- Among the phenolic acids studied, vanillic acid yielded the highest concentration under both the greenhouse and field conditions. The antioxidant activities reflecting the scavenging potency of the genotypes were generally high and correlated with their phenolic acids contents across the two environments. Also, carotenoids (lycopene and β carotene) and total flavonoid contents varied across the two environmental conditions which proved that environmental influence played a role in the production. Generally, the antioxidants studied among the genotypes were high in concentration and varied across environments. Some genotypes performed better in yield for some of the antioxidants studied and could be considered for production.
ACKNOWLEDGEMENTS
- The research team wish to appreciate the efforts of University of Ghana Forest and Horticultural Crops Research Centre (FOHCREC), Okumaning-Kade and Plant Genetic Resources Research Institute (PGRRI), Bunso both in the Eastern Region of Ghana. Also, gratitude goes to Mr. Kwadwo Obeng of the Department of Botany Laboratory, School of Biological Sciences, University of Ghana, Legon in the Greater Accra Region of Ghana for his enormous contributions during the laboratory work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML