-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2020; 10(4): 102-107
doi:10.5923/j.ijaf.20201004.03
Received: Nov. 20, 2020; Accepted: Dec. 5, 2020; Published: Dec. 15, 2020

In Vitro Effect of Extracts of Erythrina senegalensis on Two Fungal Strains Responsible for Post-harvest Losses of Papaya, Chili Pepper and Tomato
Yao Kamelé Kossonou1, Kouassi Martial-Didier Adingra2, Yao Mesmin Koffi3, Fézan Honora Tra Bi3, Kablan Tano2
1Department of Agronomic Forest and Environmental Engineering, Man University, Man, Côte d’Ivoire,
2Department of Food Science and Technology, Nangui Abrogoua University, Abidjan, Côte d'Ivoire
3Department of Sciences of Nature, Nangui Abrogoua University, Abidjan, Côte d'Ivoire
Correspondence to: Yao Kamelé Kossonou, Department of Agronomic Forest and Environmental Engineering, Man University, Man, Côte d’Ivoire,.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

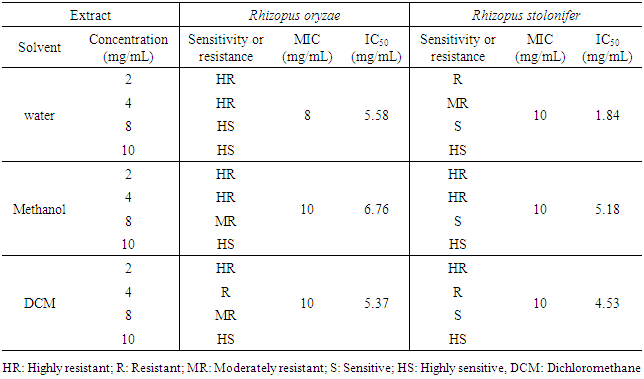
Post-harvest diseases due to the action of pathogenic fungi constitute a real problem in the marketing of fruits and vegetables, which represents an important sub-sector of the economy of Côte d'Ivoire. The conservation of fruits and vegetables has always relied in developed countries on the use of techniques that are difficult for producers to access or on the use of chemicals. Today, the search for new conservation techniques that are less expensive and concerned with the well-being of consumers is increasingly being considered. Several recent data suggest that medicinal plants with antifungal properties could represent an alternative solution for the control of these diseases. To this end, the antifungal potential of aqueous, methanolic and dichloromethane extracts of the leaves of Erythrina senegalensis was tested on Rhizopus oryzae and Rhizopus stolonifer, two fungi responsible for post-harvest diseases of papaya, chili pepper and tomato. The antifungal tests performed showed that the extracts of E. senegalensis inhibited the mycelial growth of fungal strains with minimum inhibitory concentrations (MIC) of between 8 mg/ mL and 10 mg/mL. However, of the three extracts tested, the aqueous extracts were the most active on fungi. The beneficial effect of E. senegalensis against the fungi R. oryzae and R. stolonifer leads to suggest that this plant be further explored in order to consider a formulation of biofungicides in the conservation of fruits and vegetables in Côte d'Ivoire.
Keywords: Fruits and vegetables, Post-harvest losses, Antifungal, Erythrina senegalensis
Cite this paper: Yao Kamelé Kossonou, Kouassi Martial-Didier Adingra, Yao Mesmin Koffi, Fézan Honora Tra Bi, Kablan Tano, In Vitro Effect of Extracts of Erythrina senegalensis on Two Fungal Strains Responsible for Post-harvest Losses of Papaya, Chili Pepper and Tomato, International Journal of Agriculture and Forestry, Vol. 10 No. 4, 2020, pp. 102-107. doi: 10.5923/j.ijaf.20201004.03.
Article Outline
1. Introduction
- Papaya, chili pepper and tomato crops are currently important activities in Côte d'Ivoire [1,2]. With an annual production of 32,900 tonnes, chili pepper, along with tomatoe, okra and eggplant, are the main vegetables consumed and cultivated [3,4]. Regarding papaya, the Côte d’Ivoire and Ghana are the main suppliers to the European Union markets in West Africa [1]. Despite their economic importance, the consumption of fruits (papaya, chili pepper and tomato) is considered today as an important element of public health. It provides nutrients, vitamins, antioxidant compounds and minerals essential for the proper functioning of the body [5]. Despite their economic and nutritional importance, these climacteric fruits are extremely perishable because after harvest they continue to maintain metabolic activity. Post-harvest losses are often estimated at between 30% and 40% of production and represent a considerable shortfall in the national economy. These losses are due to senescence, sweating and fungal and bacterial infections [6,7]. Fungal species such as Fusarium spp., Rhizopus spp. and Colletotrichum gloeosprioides and pathogenic bacteria such as Pseudomonas spp. and Erwinia spp., play an important role in post-harvest disease and rots of fruits [8]. In order to extend shelf life and reduce post-harvest losses, researchers used ultraviolet C irradiation [9] and several chemicals such as methylcyclopropene (1_MCP) [10], silver nitrate [11]. However, consumers are increasingly reluctant to use these products because of their residues.Other technologies, such as controlled and modified atmosphere storage, have been used to extend the shelf life of fruit but are too expensive for the individual grower [12,13]. The high cost of these conservation technologies and the reluctance of consumers towards these chemicals make it necessary to explore new conservation methods, in particular the implementation of biofungicides which are products obtained from plant extracts in order to fight against contaminating agents in fruits and vegetables.Recent studies have shown that products of plant origin are a remedy in the fight against post-harvest losses of fruits and vegetables [14,15,16]. Erythrina senegalensis DC. (Leguminosae) is an African medicinal plant [17] traditionally used against several pathologies such as gastrointestinal disorders, wounds, malaria, dysmenorrhea, pneumonia, cough, onchocerciasis, inflammation, nosebleeds, dizziness, jaundice and venereal disease [18]. The plant is also known to cure urinary schistosomiasis and eye infections [19]. In Côte d’Ivoire, pharmacological studies have shown that E. senegalensis has antibacterial and antifungal pharmacological activities [20,21,22]. Elsewhere in Africa, the antibacterial and antifungal activity of E. senegalensis has been demonstrated by Magassouba et al. [23] and Doughari [24].These authors have shown that the stem barks of this plant are active on bacteria (Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Salmonella typhi, Pseudomonas aeruginosa) as well as on fungi (Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Penicillium notatum).Given the many pharmacological properties of E. senegalensis in the treatment of human pathologies, it would be interesting to study its potential in the search for treatments against postharvest fruit diseases. Therefore, this study aims to evaluate the potential of E. senegalensis against fungi responsible for post-harvest diseases of papaya, chili pepper and tomato.
2. Material and Methods
2.1. Material
2.1.1. Collection and authentication of E. senegalensis
- Fresh leaves of E. senegalensis were collected from Bongouanou (Côte d'Ivoire). The plant was authenticated by Professor Laurent Ake-Assi of Floristic National Center (FNC) of Université Felix Houphouët Boigny (Côte d’Ivoire).
2.1.2. Selection of Fungal Strains
- The fungal strains Rhizopus oryzae and Rhizopus stolonifer are spoilage agents in the fruits of papaya, chili pepper and tomato. The fungal strains R. oryzae and R. stolonifer used were isolated from the fruits of papayas, chili peppers and tomatoes taken from fields in the municipality of Azaguié (Côte d’Ivoire). These strains were then identified using the PCR-DGGE molecular method followed by sequencing [25]. According to this study, these fungal strains were present on 99% of the fruits sampled for R. oryzae and 100% for R. stolonifer.
2.2. Methods
2.2.1. Sabouraud Culture Medium
- Sabouraud culture medium was prepared according to the method described by Alrasheid et al. [26]. Sixty-two grams of the powdered Sabouraud dextrose agar, was weighed, dispersed in 1 L of distilled water and allowed to soak for 10 min, swirled to mix then sterilized by autoclaving for 15 min at 121°C, cooled to 47°C. Lastly, mixed well and poured into petri dishes.
2.2.2. Preparation of Plant Crude Extracts
- The plant extracts were obtained from the powder of the leaves of E. senegalensis. Powder was macerated at room temperature with mechanical stirring in three solvents as follows: distilled water, methanol (99.8%), and dichloromethane (99.9%) according to solvent-to sample ratio (v/w) at 10:1. After filtration, the methanolic extract obtained was concentrated on a rotavapor (Büchi R-104, Switzerland). The methanol concentrate collected in a little water and the aqueous concentrate were then lyophilized [27]. A stock solution of 100 mg/mL concentration was prepared by dissolving 1.2 grams of lyophilized plant extract in 12 mL of dimethylsulfoxide (DMSO).
2.2.3. Antifungal Assay
- Inoculum preparationThe antifungal activity of the extracts against Rhizopus oryzae and Rhizopus stolonifer was determined by the Sabouraud agar with chloramphenicol method [15]. Both fungal strains were grown at 28°C for 48 hours to obtain a young culture. The colony of the young culture obtained was introduced into a tube containing a volume of 15 mL of Sabouraud broth with chloramphenicol. The prepared inoculum is used for the inoculations.Seeding and antifungal effect of extract on mycelial growthA volume of 15 mL of the supercooled agar was previously poured into each Petri dish. Before solidification, a volume V of the stock solution of plant extract is taken using a micropipette and homogenized in the agar, according to the concentration desired for carrying out the test. Thus, for a culture medium equivalent to 15 mL of agar, 300 μL, 600 μL, 1200 μL and 1500 μL of stock solution were added for respective equivalent concentrations of 2, 4, 8 and 10 mg/mL. A volume of 0.5 μL of the inoculum containing the germ was taken and deposited on the solidified Sabouraud chloramphenicol media and incorporated in various plant extracts for the treatments. A Control was prepared using the same process, but with the difference that it does not contain plant extracts. Petri dishes were incubated for six days in the dark at 25°C ± 2 (laboratory temperature). The parameter measured was the average diameter of the radial growth of the mycelium of each strain of fungus. For each treatment, three Petri dishes were used and the test was repeated three times. The measurements were carried out every day for six days. Then the percent mycelial growth inhibition was calculated with the average radial growth of mycelium for each concentration. This made it possible to determine the level of resistance or sensitivity of each germ for each plant extract. The percentage inhibition was calculated according to the formula and the sensitivity scale proposed by Kumar et al. [28]:I (%) = (C-T)/T ×100I (%): Percent inhibitionC: Radial growth (cm) of the controlT: Radial growth (cm) of the treatmentThe isolates were classified based on their reaction to different extracts as given below: 1) Highly sensitive (> 90% inhibition); 2) Sensitive (> 75-90% inhibition); 3) Moderately resistant (> 60-75% inhibition); 4) Resistant (> 40-60% inhibition) and 5) Highly resistant (< 40% inhibition).
2.2.4. Determination of MIC and IC50
- The determination of the minimum inhibitory concentrations (MIC) was carried out by the method of successive dilution in agar medium. This technique consists of determining the lowest concentrations at which the plant extracts totally inhibit the isolated fungal strains. It was done with the naked eye from the Petri dishes after the six days of incubation.
2.2.5. Data Analysis
- The results of the inhibition tests of radial mycelial growth of extracts of Erythrina senegalensis on the two fungal strains were expressed as percent inhibition. One-factor analysis of variance (ANOVA 1) was used to compare the means of the percentages of inhibition induced by the concentration of the extracts on the mycelial growth of the fungal strains. When a significant difference is observed between the means compared to the 5% threshold, Tukey's post-ANOVA test is performed to find out the level of difference between the means in order to rank them. The values of the minimum inhibitory concentrations (MIC) were determined using the histogram of the percent inhibitions. The analyses of variance were carried out using the XLSTAT 2020 [29] software incorporated in Excel 2010. For the determination of the concentrations which inhibit 50% of the mycelial growth of fungi (IC50), a trend curve between the concentrations of the extracts and the percentages of inhibition using Excel 2010 software.
3. Results
- The study of the effect of extracts of Erythrina senegalensis (aqueous, methanolic and dichloromethanic) on the mycelial growth of the fungal strains Rhizopus oryzae and Rhizopus stolonifer showed that the plant extracts caused inhibitions of the radial mycelial growth which are function the concentration of extracts.Figure 1 shows the percentages of inhibitions induced on radial mycelial growth of R. oryzae as a function of the concentration in the three extraction solvents. In this figure, statistical analysis shows a difference in activity from one concentration of extract to another for the three extraction solvents. In water, at 2 mg/mL and 4 mg/mL, the extract caused less than 40% inhibition of mycelial growth while at concentrations of 8 mg/mL and 10 mg/mL the percent d inhibition obtained is 100%. For the methanol and dichloromethane extracts, a percent inhibition of radial mycelial growth of 100% was obtained only at the concentration of 10 mg/mL. On this fungal strain, the aqueous extract showed more activity than the organic extracts.
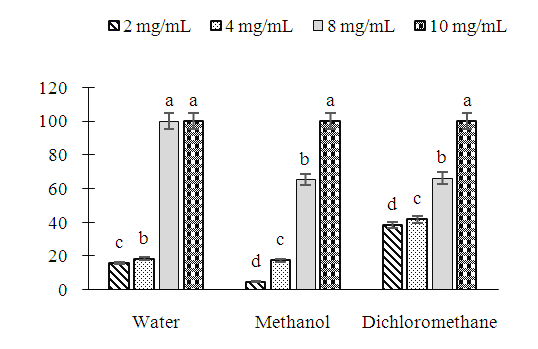 | Figure 1. Induced inhibition (%) on the mycelial growth of R. oryzae by extracts of Erythrina senegalensis as a function of the concentration |
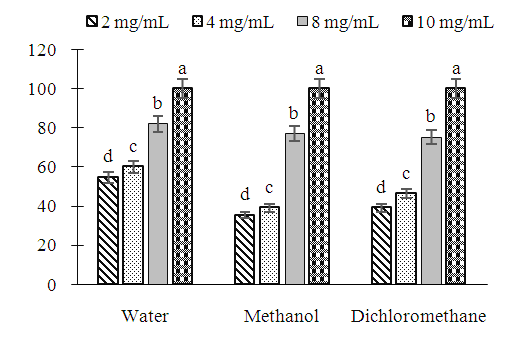 | Figure 2. Induced inhibition (%) on the mycelial growth of R. stolonifer by extracts of Erythrina senegalensis as a function of the concentration |
|
4. Discussion
- The study of the effect of extracts of Erythrina senegalensis on the fungal strains Rhizopus oryzae and Rhizopus stolonifer has shown that this plant has an inhibitory activity on the growth of fungi. Indeed, these two fungal strains were very sensitive to the aqueous, methanolic and dichloromethane extracts of the plant. The antifungal activities of E. senegalensis leaves extracts against these fungi responsible for post-harvest rots in papaya, chili pepper and tomato are reported here for the first time. Several studies carried out using E. senegalensis have shown that this plant has many biological properties in cases of human pathologies. For example, antibacterial activities against Staphylococcus aureus, Streptococcus pyogenes, Escherichia coli, Salmonella typhi and Pseudomonas aeruginosa have been demonstrated by Doughari [24]. Likewise, the work of Koné et al. [30] made it possible to demonstrate the activities of this plant on resistant strains of Streptococcus pneumoniae. In addition, the extracts of this plant have been active on fungi such as Aspergillus flavus, Aspergillus fumigatus, Candida albicans and Penicillium notatum [24]. The various pharmacological properties of E. senegalensis against these human bacteria and fungi may explain its activity against the fungal strains Rhizopus oryzae and Rhizopus stolonifer. On these fungals, the same author has shown that the methanolic extract of E. Senegalensis had MIC values between 10 mg/mL and 30 mg/mL. Other authors like Masangwa et al. [36] obtained with the aqueous extract of Chlorophytum comosum an MIC of 12.5 mg/mL on Colletotrichum dematium, a fungus responsible for post-harvest fruit rot. The different MIC values observed could be explained either by the nature of the plant used, or by the extraction solvent or by the fungal strain tested. Although not having worked on the same fungal strains, these results nevertheless show that the extracts of this plant had a beneficial effect on the fungal strains studied.Phytochemical screening of E. senegalensis leaves extracts showed that they contained saponins, terpenoids, steroids, tannins, flavonoids, phenols and alkaloids [31]. Several authors have shown that secondary metabolites are responsible for the biological activities of plant extracts [32,33,34,35]. The presence of these secondary metabolites explains the biological activities of this plant in this study and its traditional use as a medicinal plant [18]. The results also showed that the aqueous extract of E. senegalensis was more active on the strains than the organic extracts. The difference in activity between aqueous and organic extracts could be explained by the nature of the molecules contained in each of the types of extracts. Indeed, according to Ullah et al. [37] and Wakeel et al. [38], during the extraction, the phytomolecules are distributed among the solvents according to their polarity and their solubility. It could be deduced that the antifungal substances contained in plant extracts are more soluble in water than in other organic solvents. However, it is sometimes recognized that the potency of medicinal plants can be enhanced by using organic solvents [39].
5. Conclusions
- This work was developed to search for natural products that are alternatives to chemicals used in the control of fungal pathogens responsible for rotting tropical fruits (papaya, chili pepper and tomato). Thus, the antifungal tests carried out using extracts of Erythrina senegalensis against Rhizopus oryzae and Rhizopus stolonifer showed that these plant extracts were active on these two phytopathogenic agents. The activity of plant extracts on these fungal strains suggests considering a formulation of natural biofungicides concerned with the well-being of consumers in the conservation of post-harvest products.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML