-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2020; 10(2): 41-48
doi:10.5923/j.ijaf.20201002.01

Evaluation of External and Internal Egg Quality Traits of Indigenous Sakini Chicken in Different Generations of Selection
Saroj Sapkota 1, 2, Mana Raj Kolakshyapati 3, Naba Raj Devkota 4, Neena Amatya Gorkhali 1, Nirajan Bhattarai 2
1Animal Breeding Division, Nepal Agricultural Research Council, Khumaltar, Lalitpur, Nepal
2Department of Animal Breeding and Biotechnology, Faculty of Animal Science Veterinary Science and Fisheries Agriculture and Forestry University, Rampur, Chitwan, Nepal
3Department of Animal Breeding and Biotechnology, Institute of Agriculture and Animal Science, Tribhuvan University, Kathmandu, Nepal
4Directorate of Research and Extension, Agriculture and Forestry University, Rampur, Chitwan, Nepal
Correspondence to: Nirajan Bhattarai , Department of Animal Breeding and Biotechnology, Faculty of Animal Science Veterinary Science and Fisheries Agriculture and Forestry University, Rampur, Chitwan, Nepal.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Indigenous Sakini chicken is the principal breed in Nepal. Present study was conducted mainly aiming at comparing external and internal egg quality traits in four different generations (G0, G1, G2 and G3) of Sakini chicken and determining relationships among these traits. Total of 154 eggs (G0: 30, G1: 44, G2: 40, G3: 40) were evaluated for external egg traits like egg weight, egg length, egg breadth, shell thickness, shell weight and internal egg traits like yolk weight, yolk height, yolk diameter, albumen weight, albumen height, albumen diameter. The data were recorded and analysed using GenStat 19 edition software. There was significant difference in almost all traits of external and internal traits of egg except egg shell quality, yolk to albumin ratio and yolk percentage. Encouragingly, we had observed an increasing trend for each trait specially Haugh unit, a measure for better quality of egg protein in every generation indicating that selection to be continued unless the uniform performance is demonstrated in the population. Positive and significant (p<0.01) correlations (r = 0.44-0.92) were observed between egg quality traits under study. Findings suggested that selection brings genetic improvement in most of the egg quality traits of indigenous Sakini chicken. However, continuous selection practices to be employed in successive generations to exploit the maximum genetic potential in Sakini chicken.
Keywords: Sakini, Indigenous, Chicken, Egg quality traits
Cite this paper: Saroj Sapkota , Mana Raj Kolakshyapati , Naba Raj Devkota , Neena Amatya Gorkhali , Nirajan Bhattarai , Evaluation of External and Internal Egg Quality Traits of Indigenous Sakini Chicken in Different Generations of Selection, International Journal of Agriculture and Forestry, Vol. 10 No. 2, 2020, pp. 41-48. doi: 10.5923/j.ijaf.20201002.01.
Article Outline
1. Introduction
- Chicken farming is one among the fastest growing livestock commodity in Nepal. It occupies a fundamental position in current Nepalese economy with the share of 9% in Agriculture GDP and contribution of 17.4% of total meat production (DLS, 2018) and has evolved from subsistence farming to an extremely sophisticated business oriented enterprise. This transformation was indebted to huge investment in overall management practices by both government and private organizations. The share of backyard chicken (indigenous) in total poultry production is about 50% and the trend is increasing (DLS, 2018). Indigenous chicken has very important socio-economic role in rural communities providing them animal protein, generation of extra cash incomes and religious considerations. Sakini chicken is the principal indigenous breed of Nepal. This is found in all agro-ecological zones and distributed across the country. Sakini chicken is well-known as the appropriate backyard poultry breed in resource poor environment and raised under traditional scavenging management system. They have adaptive potential to the prevailing environment, disease and other stresses (Chebo and Nigussie, 2016). Consumers usually prefer products (meat and eggs) of indigenous chicken to exotic ones because of their taste, flavor and nutrition. In spite of their significant roles, their low performance in their egg and meat production masked their contribution to uplift the living standards of their owners and contribute to rural development (Markoset al., 2017). Beside about traits, the storage and hatchability traits of egg could play an important role for the acceptability to the consumer preference,. Hence, the present study was designed to improve the external and internal egg quality traits through selection over four generations (G0-G3). In this study, we compared the egg quality traits of Nepalese Sakini chicken improved over the generations and determined relationships among these traits.This study aids to ensure improvement of chicken productivity, sustainable utilization and conservation of indigenous chicken genetic resources to respond the demand of chicken products. However, no research or very limited work has been done regarding the evaluation of egg quality traits of indigenous chicken.
2. Methodology
- Description of experimental locationThe study was carried out under Animal Breeding Division, NARC in poultry unit of Swine and Avian Research Program, NARC, Khumaltar from March 2015 to December 2018 for four consecutive generations (G0, G1, G2 and G3). The poultry unit at SARP lies at a mean elevation of about 1350 masl. Yearly average temperature in the Khumaltar is 15-20°C and receives yearly average rainfall of 2000-2400 mm. Experimental birds and their management All four generations of indigenous Sakini chicken were reared in deep litter pens and fed conventional starter, grower and layer rations. Altogether 269 birds were evaluated from parent to third generations where 180 hens were sired by 89 cocks. A lighting schedule of 16 h/day was applied during laying period. Standard procedure with respect to preventive vaccination and medication were followed during the study period. The eggs were collected at 40 weeks of age and data for egg quality were recorded on the same day of collection. Total of 30, 44, 40 and 40 eggs were evaluated from parent base population (G0) and three consequent generations (G1, G2 and G3), respectively. Measurement of external egg characters The individual egg for each generation were weighed using digital balance to the nearest of 0.01 gm accuracy. The length (L) and breadth (B) of egg were measured with the help of digital Vernier calipers and shape index was calculated as the ratio of breadth to length times 100 as suggested by Anderson et al. (2004). Surface area (cm2) of the egg was calculated from L and B using formula (3.115-0.0136*L+ 0.0115*B) L*B as suggested by Narushin (2005). The egg shell Breaking Strength (BS, g) of each egg was computed from its Egg Weight (EW) using the formula (50.86*EW^0.915) as suggested by Arad and Marder (1982). Similarly, the values of the egg length (L) and egg breadth (B) were used to determine the egg volume (V, cm3) using Hoyt (1979) equation (V=Kv*LB2) where the estimated volume coefficient (Kv=0.507) is applicable to all eggs which are not very pointed.. The shells were kept in the open air for 24 hours for drying. All the dried shells were weighed with the help of a digital balance. The shell weight was divided by the egg weight to get the shell ratio. The thickness of four pieces of egg shells, one each from the two ends (broad and narrow end) and two from the middle of the eggs, were measured to the nearest of 0.01 mm with the help of screw gauze micrometer and averaged. Measurement of internal egg characters The length and width of the albumen and yolk were measured in mm with the help of a vernier caliper (least count 0.01 mm). The height of the albumen and yolk were measured at the top by spherometer on a flat table (level for the table is maintained using standard procedure). The height of the albumen was measured at 3 or 4 locations and averaged. Haugh unit (H.U.), a measure of egg protein quality was calculated by using the formula 100 log (H+7.57−1.7EW0.37) given by Haugh (1937) where, H is albumen height in millimeters and EW is observed weight of the egg in grams. Albumen and yolk indices were estimated in percentage, taking the ratio of their respective heights to the average of breadth and length as suggested by previous workers (Kul and Seker, 2004). Albumen weight (g) was calculated as Egg Weight - (Yolk Weight + Shell Weight). Albumen and yolk ratio was calculated taking the individual weight as the percentage of total egg weight. Yolk diameter was estimated as the average of yolk length and breadth. Yolk to albumen ratio was calculated as weight of yolk to the weight of albumen. Yolk index (%) was calculated as the ratio of yolk height to yolk diameter times 100.Statistical AnalysisAll the egg quality data were managed using Ms-Excel spreadsheet. Least square means with standard errors of mean (LS±SEM) were calculated for all the egg quality traits using GenStat 19th edition software (VSN International, 2018).
3. Results and Discussion
- The results on comparative internal and external traits of egg quality of Sakini base population (G0) and their subsequent generations (G1, G2, G3) after selective breeding is presented in Table 1.
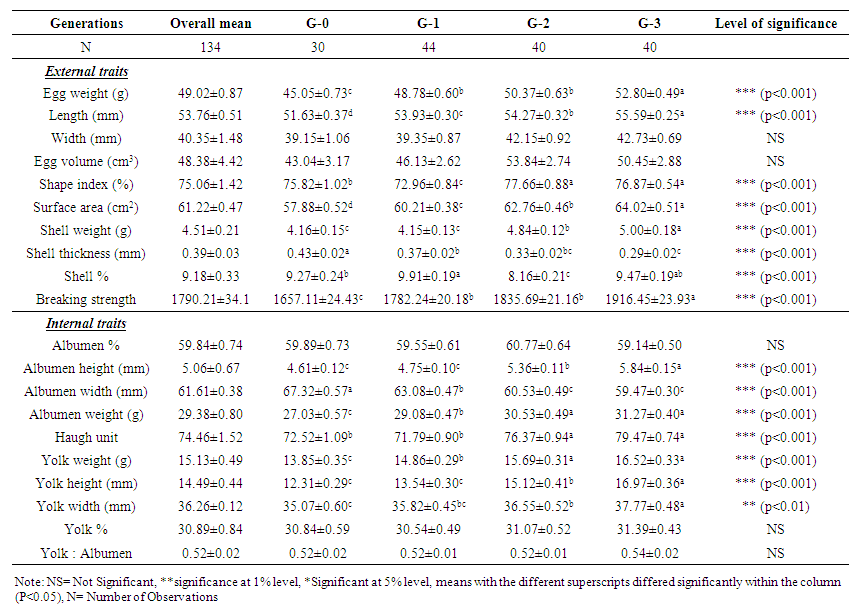 | Table 1. Effect of selection on egg quality traits in different successive generations in Indigenous Sakini chicken (LS mean ± SE) |
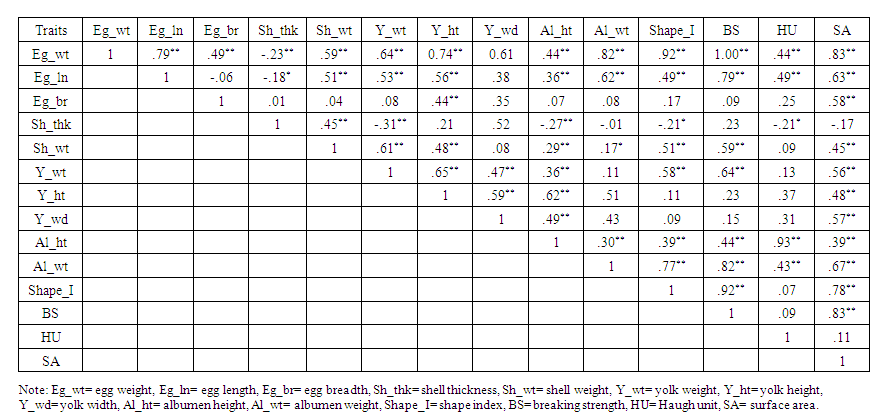 | Table 2. Correlation among the external and internal egg quality traits of Indigenous Sakini chicken under intensive management |
4. Conclusions
- The results of this study provide support to suggest that genetic improvement in most of the egg quality traits of indigenous Sakini chickens could be achieved through selection practice in subsequent generations. Egg external and internal quality traits like egg weight, length, shape index, surface area, shell thickness, shell percent, shell ratio, breaking strength, albumen and yolk related traits and most importantly Haugh Unit mainly related to egg protein quality plays significant roles. Similarly, egg weight has medium to high correlation with all egg quality traits except shell thickness.
Ethical Statement
- I testify that my article submitted to International Journal of Agriculture and Forestry entitled “Evaluation of external and internal egg quality traits of indigenous Sakini chicken in different generations of selection” Authors: 1) this material has not been published in the whole or in part elsewhere; 2) the manuscript is not currently being considered for publication in another journal; 3) We have been actively involved in substantive work leading to the manuscript and will hold ourselves responsible for content. Date: 25/03/2020
ACKNOWLEDGEMENTS
- The authors acknowledge the financial support for research from Nepal Agricultural Research Council (NARC), Nepal and Feed the Future Innovation Lab for Livestock Systems, USAID, University of Florida for supporting tuition fee at Agriculture and Forestry University (AFU), Rampur, Chitwan, Nepal. We like to extend our gratitude to Swine and Avian Research Program for providing the research facilities.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML