-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2019; 9(1): 32-37
doi:10.5923/j.ijaf.20190901.03

Effect of Light and Stump Height on Coppice Regeneration of Umbrella Tree (Maesopsis emineii Engler)
Sharon Fombasso1, Titus Fondo Ambebe2
1Department of Development Studies, Pan African Institute for Development – West Africa, Buea, Cameroon
2Department of Crop Production Technology, College of Technology, The University of Bamenda, Bambili, Cameroon
Correspondence to: Titus Fondo Ambebe, Department of Crop Production Technology, College of Technology, The University of Bamenda, Bambili, Cameroon.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Shoots of young Maesopsis emineii are vulnerable to decapitation by browsing animals. Re-sprouting probability of the residual stumps and growth of sprouts are influenced by several factors. To investigate the effects of light and stump height on sprouting of stumps, and survival and growth of new shoots, stumps of 6-month old seedlings of the species were exposed to two light levels (50% and 100% sunlight) and three stump heights (0, 4, and 6 cm) for four months. The treatments were laid out in a completely randomized block design arranged in a split-plot with light as the whole plot and stump height as the sub-plot. The data were subjected to analysis of variance. The full sunlight treatment increased percentage of stumps sprouted but reduced height of the tallest sprout. The 0 cm cut height decreased percentage of stumps sprouted, number of sprouts per stump, percentage survival of sprouts, height and number of leaves of the tallest sprout. There was no significant interactive effect of treatments on any parameter. The findings suggest that tending low-cut stumps may be less beneficial than high-cut counterparts for coppice regeneration of Maesopsis emineii. In addition, height growth of coppice shoots can be precipitated by shading. Thus, light intensity and stump height should be taken into consideration in the design of silvicultural options for regeneration of the species from seedling stumps.
Keywords: Coppice Silviculture, Light Intensity, Musizi, Sprout Growth, Tropical Forests
Cite this paper: Sharon Fombasso, Titus Fondo Ambebe, Effect of Light and Stump Height on Coppice Regeneration of Umbrella Tree (Maesopsis emineii Engler), International Journal of Agriculture and Forestry, Vol. 9 No. 1, 2019, pp. 32-37. doi: 10.5923/j.ijaf.20190901.03.
Article Outline
1. Introduction
- Maesopsis emineii is an early successional semi-deciduous tree belonging to the family Rhamnaceae. The tree grows to a height of 30 m (- 45 m) with a clear bole up to 10 m, and a diameter of 10-180 cm [1]. Umbrella Tree, as it is commonly called, is native to Africa, occurring naturally in a band across Western, Central and Eastern Africa between 8°N and 6°S. Maesopsis emineii is mainly found in humid forests, colonising cleared areas and flourishing as secondary growth. It is very common in the border zone between savannah and forest [2]. Its biophysical limits are 700-1500 m altitude, 22-27°C mean annual temperature, and 1200-3000 mm mean annual rainfall [3]. The multipurpose tree is an important component of the Western Highlands forests of Cameroon. The strong and durable timber is used for poles, plywood, boxes, millwork, and lumber construction while the seeds are a source of edible oils. Leaves with 35% dry matter content constitute an important source of animal forage. Furthermore, the root and bark are of medicinal value [4]. Maesopsis emineii is a fast growing species, attaining up to 3 m in height and 55 mm in diameter per year when young [1]. Due to the high growth rate, it is often planted for fuelwood and as a shade tree in agroforestry systems. In addition, the ability of this pioneer species to aggressively colonize grasslands makes it suitable reforestation material [4, 5]. Maesopsis emineii is becoming increasingly popular because of its fast maturation and production of more wood per unit area when compared to other tree species [6]. The sustainable supply of these and other benefits depends on the proper management of the species.With a prolific seed producing habit and tolerance to a wide range of site factors, natural regeneration is a suitable method for propagation of Maesopsis emineii [7]. Seed dispersal of this insect-pollinated tree is aided by birds, bats, monkeys, and chimpanzees [8, 9]. Artificial regeneration by direct seeding can be effected, with best results obtained after soaking seeds in cold water for 2-3 days or concentrated sulphuric acid for 20 minutes [4, 10]. However, the appealing nature of the foliage has made seedlings highly susceptible to browsing animals. This and other disturbance events often result in breakages at various positions on the stem. Like other broad-leaved tree species of moist tropical forests, however, there is potential for recovery via re-sprouting of stumps of the decapitated plants in a process that is known as coppicing [11, 12]. The new shoots arise from dormant buds on the side of the stump or from adventitious buds developing in the cambial layer beneath the bark [13].Coppicing ability can be influenced by several factors. For instance, high amounts of far-red light, as found in shaded environments [14], inhibit the initiation of bud outgrowth but promote the subsequent elongation of the bud after initiation [15-17]. Similarly, responses of coppicing and coppice shoots to cutting method [18-21], season of cutting [19, 20, 22, 23], stump age [19, 24, 25], and stump height and diameter [18, 24, 26, 27, 28] have been examined. Results vary from one species to another, suggesting that a species specific, rather than holistic, approach is needed for the management of stumps and coppice shoots for advance regeneration. There is a general lack of information on the reaction of Maesopsis emineii to these factors. Such a knowledge gap poses a hindrance to decision making on whether to tend or discard the truncated seedlings on a regeneration site as well as to coppice management. This study was aimed at exploring the effects of light and stump height on coppice regeneration of the species.
2. Materials and Methods
2.1. Study Site
- The experiment was conducted at the Reforestation Task Force (RETAFO) nursery located in Bamenda III Sub-Divison of the North West Region of Cameroon. Bamenda is situated between latitude 5.9586 and longitude 10.1475 at an altitude of 1250 m. It is the administrative headquarter of the North West Region. The site is characterized by a rainy (April to October) and dry (November to March) season, a mean annual temperature of 21.5°C, and a mean annual precipitation of 2145 mm [29]. March and July with an average temperature of 23.0°C and 20.1°C, respectively, is the warmest and coldest month of the year. The driest month is January with 9 mm of precipitation while the wettest is September with an average of 383 mm [29]. During the months of August, September, October, and November 2018 when the experiment took place, mean monthly temperature was 20, 21, 22 and 23°C and rainfall was 678.4, 794.4, 324.7, and 58.6 mm, respectively [30].
2.2. Plant Material
- Seeds of Maesopsis emineii were soaked in cold water in a sealed plastic bag and placed under ambient environmental conditions for two days. The seeds were then sown to a depth of 3 cm in a seedbed. The sand and soil mixture that constituted the germination substrate was kept constantly moist. At the age of six weeks, seedlings were transplanted individually into polythene bags filled with a 1:1 (v/v) soil:sawdust mix. The seedlings were irrigated as necessary. At the age of six months, the stem of each seedling was clipped off at one of three predetermined distances aboveground to produce stumps of different heights.
2.3. Experimental Design
- Treatments comprised of two light levels (50% and 100% sunlight) and three stump heights (0, 4, and 6 cm). The lower light treatment was achieved by covering the stumps with a forest green knitted shade fabric with 50% UV block (Coolaroo, USA). The experiment followed a completely randomized block design arranged in a split-plot with light as the whole plot and stump height as the sub-plot. The seedlings in the 0 cm stump height treatment were decapitated at the root-collar. The cuts on all seedlings at each stump height were made straight and transversely using secateurs. There were ten stumps per stump height × light treatment in two replications. Treatments were initiated on August 1 and terminated on November 30, 2019.
2.4. Data Collection
- The stumps were monitored daily for sprouting. The percentage of stumps with sprouts was determined when the number of stumps that sprouted was no longer increasing. Three stumps bearing sprouts were randomly selected from each treatment and replication from which data were elicited. The number of sprouts per stump was counted. Height and diameter of the tallest sprout were measured at the end of the experiment. The number of leaves of the tallest sprout was also recorded. Survival was calculated as the ratio of the number of stumps that retained live sprouts at the end of the experiment to the total number of the stumps that had sprouted.
2.5. Data Analysis
- Analysis of variance (ANOVA) was used to test for main and interactive effects of treatments. When the F-value was significant (p ≤ 0.05) for a given parameter, treatment differences were further investigated using the least significant difference (LSD) statistic. Prior to all analyses Levene’s Test was used to test for homogeneity of variance.
3. Results
3.1. Sprouting Ability
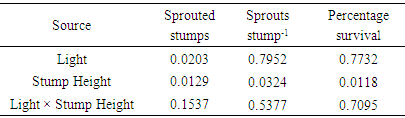
- There was a significant effect of light and stump height on percentage of stumps with sprouts (Table 1). Percent sprouting increased from the 50% to 100% sunlight environment (Figure 1). Similarly, values increased from the 0 cm to the 4 cm and 6 cm stump height treatments which showed similar responses of the trait (Figure 1).The number of sprouts per stump and survival responded to stump height in a similar manner to sprouting percentage. In contrast, however, the latter trait was unaffected by light regime (Table 1, Figure 1). None of the three sprouting attributes was significantly influenced by factorial combinations of treatments (Table 1).
|
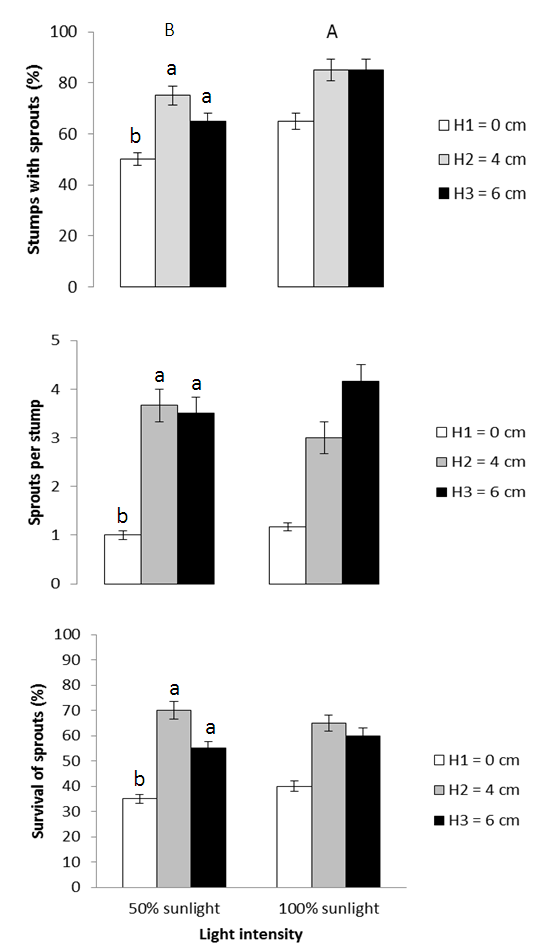 | Figure 1. Effects of light and stump height on sprouting. The upper- and lower-case letters above the bars represent the effect of light and stump height, respectively |
3.2. Sprout Growth
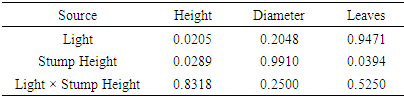
- Height of the tallest sprout was significantly affected by light and stump height but not by interactions of the treatments (Table 2). The parameter was significantly augmented by 50% sunlight (Figure 2). On the other hand, they declined from the 4 cm and 6 cm to the 0 cm stump height. There was no significant difference between the upper two stump height treatments for this trait (Figure 2).Number of leaves per sprout responded to stump height with the same trend as height of tallest sprout but was unaffected by either light or its interaction with stump height (Table 2, Figure 2). Furthermore, the ANOVA did not detect any significant main or interactive effect of treatments on the diameter of the tallest sprout (Table 2, Figure 2).
|
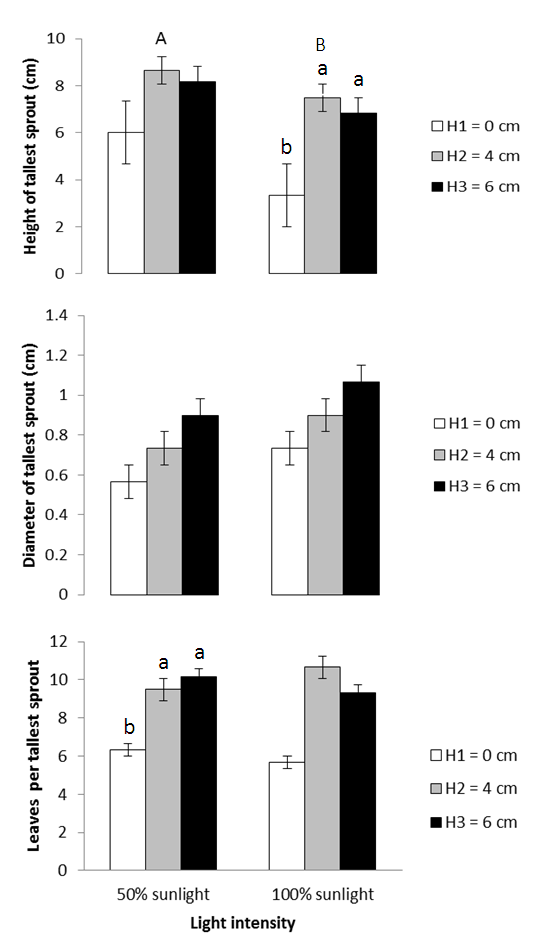 | Figure 2. Effects of light and stump height on sprout growth. The upper- and lower-case letters above the bars represent the effect of light and stump height, respectively |
4. Discussion
4.1. Sprouting Ability
- The halved sunlight treatment was meant to mimic the light climate in forest understory. As light passes through plant canopies, there is selective absorption of red light by foliage. Since the vegetation is transparent to far-red wavelengths, major reductions in the ratio of red to far-red light (R:FR) are commonly observed within canopies. With the exception of far-red light, total irradiance throughout the spectrum may decline markedly below the canopy when compared to direct sunlight [31]. The findings of this study on the light response of Maesopsis emineii coppicing potential are consistent with the conclusion of [32] that the sprouting ability of stumps is better in strong light than in shade. Low R:FR can inhibit bud outgrowth by favouring the accumulation of abscisic acid in the buds [33]. The relatively low sprouting probability in the 50% than full sunlight treatment may be ascribed, at least in part, to the R:FR effect.Our data on the effect of stump height on percent sprouting conforms to previous findings in Acer spicatum, Betula papyrifera, and Prunus pensylvanica [34]. As also observed by [35] in Eucalyptus microtheca, a rapid decay of low cut stumps was implicated in the response. According to [24], cutting treatments that promote moisture build-up on the cut surface may enhance the accumulation and activity of decay causing microorganisms with the outcome that sprouting success is compromised. Maesopsis emineii wood is highly vulnerable to decay when in contact with the ground or continual moisture [4]. The cutting of the stem at right angle to its vertical axis and at about ground level for the 0 cm cut height treatment created a platform for maximum water retention on the cut surface. The decaying of the stumps also resulted in minimal survival of sprouts emerging from the stumps of lowest cut height, in agreement with [35].Several researchers have observed a positive correlation between number of sprouts and stump height [22, 24, 27, 36-38]. The causal effect of stump height is an expression of its influence on the number of buds present. Low cuts may not leave a sufficient number of buds on the stump [39]. Consequently, relatively fewer buds in the restricted vicinity of the root-collar accounted for the lower production of shoots at the 0 cm cut height in Maesopsis emineii. Besides, the number of dormant buds which give rise to sprouts may also have been reduced due to their death in the decaying stumps [40, 41]. In Alnus rubra, the suppressive effect of the 0 cm cut height treatment on number of sprouts was offset by branch sprouts that were additionally present in taller stumps; that was in sharp contrast to the low-cut treatment where coppice shoots developed exclusively as stump sprouts around the root-collar [24].
4.2. Sprout Growth
- The beneficial effect of the high-cut stump heights on sprout growth, as documented here, has been observed in other species. For instance, sprout length of Alnus rubra was reported to increase with stump height [24]. Evidence of a positive response of sprout height and unresponsiveness of sprout diameter to stump height in Acer spicatum, Betula papyrifera, and Prunus pensylvanica is documented [34]. For Alnus nepalensis, Serixia khasiana, Quercus dealbata, and Quercus griffithii [42], number of leaves per sprout and increase in leaf number were positively correlated with stump height. The poor performance of the sprouts emerging from the low-cut stumps may be attributed to inadequate food reserves to support their growth. It is important to highlight that no nutrient fertilization was done during the 4-month duration of the experiment. Consequently, growth would have depended entirely on stored reserves when soil nutrients got depleted. On the other hand, it has been hypothesized that the dense growth of sprouts from stumps of greater heights can shield vegetation at lower strata from incoming solar radiation, leading to poor growth of sprouts arising from low-cut stumps [42]. Such was, however, not the case here as the sprouts from the taller stumps were still quite small and, besides, any possibility of shading was eliminated by ample spacing of the polythene bags. The increase in sprout height by the 50% sunlight treatment is a typical morphogenetic reaction to shade [43]. It is triggered by elevated endogenous auxin levels in response to low R:FR via de novo synthesis [44].
5. Conclusions
- The findings of this study indicate that the coppicing power of damaged Maesopsis emineii seedlings, and the survival and subsequent growth of new shoots is determined by stump height. In other words, tending low-cut stumps may not be as beneficial as high-cut counterparts for coppice regeneration of the species. In addition, height growth of coppice shoots can be precipitated by shading. Thus, light intensity and stump height should be taken into consideration when designing silvicultural options for its regeneration from stumps. However, the maximum stump height for optimum coppicing behaviour of young Meisopsis emineii plants of different ages needs to be elucidated.
ACKNOWLEDGEMENTS
- We are grateful to Mr. Emile Berinyuy and the management team of RETAFO Cameroon for technical assistance. This study was made possible thanks to a Presidential Grant of the Cameroonian Ministry of Higher Education to T.F. Ambebe.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML