-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2019; 9(1): 16-31
doi:10.5923/j.ijaf.20190901.02

Effect of Different Feed Additives on Growth Performance and Production in Livestock
Komar Akbar Hasan Al-Jaf, Yaser Khorram Del
Animal Production Department, College of Agriculture, Garmian University, Kalar, Iraq
Correspondence to: Komar Akbar Hasan Al-Jaf, Animal Production Department, College of Agriculture, Garmian University, Kalar, Iraq.
| Email: |  |
Copyright © 2019 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

As of late, the expanding worry about antibiotic-related issues, proposing feed additives as the most significant options for anti-infection agents and a standout amongst the best strategies to lessen feed costs in domesticated animals. A few feed additives might be successful in improving domesticated animals growth, however the fundamental test with these added substances is the way that outcomes acquired so far have been conflicting. For the most part, the vast majority of these added substances are viewed as safe for addition in feed; in any case their toxicity towards livestock must be deliberately checked, particularly concerning more up to date substances. The goal of this study is to give a review over the additives that are accessible for consideration in diets for domesticated animals and furthermore to incorporate the latest outcomes from growth performance and digestibility studies that are accessible.
Keywords: Feed additives, Antibiotics, Diets, Digestibility
Cite this paper: Komar Akbar Hasan Al-Jaf, Yaser Khorram Del, Effect of Different Feed Additives on Growth Performance and Production in Livestock, International Journal of Agriculture and Forestry, Vol. 9 No. 1, 2019, pp. 16-31. doi: 10.5923/j.ijaf.20190901.02.
Article Outline
1. Introduction
- The livestock sector is a highly dynamic and evolving system in response to rapidly increasing demand for livestock products (such as meat, milk, eggs and fish), largely driven by increasing human population growth, income growth and urbanization (Thornton, 2010). This increase in demand and the resulting livestock sector’s vigorous growth has made current production systems challenging. On the other hand, the increased awareness of potential negative effects of including antibiotic growth promoters in diets fed to livestock has resulted in an increased interest in producing animals without using antibiotic growth promoters. However, by eliminating antibiotic growth promoters from diets fed to newly weaned animals, disease problems may be increased and growth performance may be reduced (Liu et al., 2018). Nevertheless, to avoid the negative effects of removing antibiotic growth promoters from diets for livestock, changes in management and nutritional strategies may be required (Kil & Stein, 2010). One of the promising nutritional strategies is using different feed additives. The purpose of using feed additives is to improve the digestive and production efficiency by lowering the prevalence of pathogens and by reducing the impact of livestock on the environment (Yirga, 2015). In the present contribution, some of the additives that may be used to promote rumen fermentation, and consequently improves animal health, performance and quality of the livestock products will be discussed. The objective of the review is to provide an overview over the additives that are available for inclusion in diets for livestock and also to include the believed mechanisms for each additive as well as the most recent results from growth performance and digestibility experiments that are available.
2. Acidifiers
- Acidifier feed added substances are viewed as imperative to advance rumen fermentation, and thusly improves livestocks health, performance and quality of the livestocks products. Acidifiers are frequently utilized as options in contrast to antibiotic development advertisers due to their capacity to make a great intestinal condition for advantageous microorganisms which may result in expanded nutrient edibility, expanded growth performance, and decreased ldiarrhea. Dietary acidifiers might be natural or inorganic acids or salts of acids (Papatsiros & Billinis, 2012). The most commonly used organic acids include formic acid, fumaric acid, lactic acid, and citric acids and a comprehensive review of the effects of these acids is available (Suiryanrayna & Ramana, 2015). Inorganic acids commonly used in diets include hydrochloric acid, sulfuric acid, and phosphoric acid. Positive responses to supplementations of diets with phosphoric acid and hydrochloric acid have been reported (Mahan et al., 1996), although a lack of a positive response to hydrochloric acid has also been observed (Kil et al., 2011). Salts of acids also have been used as acidifiers for pigs and these salts include calcium-formate, potassium-diformate, sodiumdiformate, and sodium-fumarate. Positive effects of calcium-formate on growth performance and diarrhea scores were reported (Bosi et al., 2007), but in general, calcium-formate is not as efficient as potassium-diformate (Li et al., 2008a). Acids used as feed additives are predominantly natural products with minimum level of toxicity (Kirchgessner and Roth, 1988). Some of the important acidifying substances which are currently used by dairy farmers like di-carboxylic organic acids e.g. aspartate, malate, and fumarate have been evaluated as feed additives because they reduce methanogenesis byacting as ‘Hydrogen sink 'during their conversion to propionate (Newbold & Rode, 2006). Nonetheless, notwithstanding numerous long stretches of research, the precise method of activity of dietary acidifiers has not been completely explained, however the following mechanisms have been proposed: 1) a diminished or settled gastric PH may prompt expanded pepsin activity; 2) an adjustment and modification of the gut microbiota may hinder pathogenic bacterial action; and 3) acidifiers may improve nutrient absorbability in the small and large guts bringing about expanded nutrient maintenance (Kil et al., 2011; Papatsiros & Billinis, 2012).
3. Minerals
- In animal nutrition, inorganic compounds are most frequently called minerals. According to the relative amounts needed in the diet minerals can be divided into two groups, namely macro-minerals and micro-minerals or trace elements. Another criterion by which minerals can be assigned to the macro-minerals or trace elements is the amount in which they appear in the organism. Minerals required in amounts more noteworthy than 100 mg/kg of feed are called macro minerals though minerals required in littler amounts are called smaller micro minerals or trace minerals. In contrast trace elements, with the exception of iron, exhibit mean tissue concentrations lower than 0.05 g/kg (50 mg/kg) (Mosenthin et al., 2006).Essential trace elements (e.g. Fe, Zn, Mn, Cu, I, Se, Co, Cr, Mo, Ni) are the most important inorganic feed additives in the nutrition of laboratory animals, livestock and companion animals. Their function as integral parts of diverse proteins, enzymes and hormones involved in fundamental metabolic and protective (anti-oxidative) processes make them indispensable. Low native dietary concentrations, as well as the existence of inorganic and organic inhibitors for some of the trace elements in natural dietary sources, make it necessary to supplement animals with trace minerals up to requirement level, especially in view of the increased performance of productive livestock in recent years.
3.1. Zinc
- Zinc is a segment and activator of a few metallo-enzymes, and has a noteworthy capacity underway and emission of hormones. It additionally assumes a job in skin and wound recuperating and in the integrity of the immune system (McDowell, 1992). Nursery pigs typically expect 80 to 100 mg/kg of Zn (van Heugten et al., 2003) and inadequacy of Zn in weanling pig diets prompts development impediment, loss of appetite, skeletal variations from the norm, and hyper-keratinization of the skin called Parakeratosis (Prasad et al., 1971). Signs of dietary zinc deficiency in farm animals depend on the species, but there are similarities between the symptoms of a subclinical and a clinically manifested zinc deficiency. Pigs are more susceptible to a clinically manifested zinc deficiency than ruminants.The organic instrument of Zn in upgrading development execution focuses might be identified with its capacity in the intestinal integrity and morphology in weanling pigs (Pearce et al., 2015). High Zn admission improves the intestinal morphology of weaning pigs, expanding the villous stature and the villous tallness to crypt profundity proportion (Xia et al., 2017; Zhu et al., 2017) and diminishes crypt profundity in the small digestive tract of weaned pigs (Zhu et al., 2017). In cattle, an early sign of zinc deficiency is excessive salivation, which may be caused by a reluctance to swallow the large amount of saliva that is normally produced. A mild zinc deficiency in cattle results in lowered weight gain (Goetsch et al., 1991). In lambs zinc deficiency results in lack of appetite, reduced growth, loss of wool, swelling around the eyes and hooves, excess salivation, general listlessness, impaired growth of the testes and cessation of spermatogenesis (Kendall & Telfer, 2000). Feeding ewes low-zinc diets during pregnancy leads to a reduced survival rate of newborn lambs (Apgar et al., 1993), while reabsorption of the fetus, delivery of mummified deformed lambs and abortions have also been reported as a consequence of dietary zinc deficiency. The zinc requirement for sheep is 20–35 mg/kg total diet and the maximum tolerable level is 750 mg/kg diet, above which symptoms of excess zinc can occur, such as induction of copper deficiency (NRC, 1985).
3.2. Copper
- Copper is a basic segment of a few metallo-enzymes including cytochrome oxidase and lysyl oxidase, and is engaged with oxidation-reduction reactions, movement of oxygen and electrons, and assurance against oxidative stress (Hill, 2013). In general, copper deficiency can occur in all mammalian species, however ruminants are perhaps the most susceptible. An early sign of copper deficiency in cattle is loss of hair pigmentation, particularly around the eyes. Scour is a clinical sign of copper deficiency that seems to be unique to ruminants, though the pathogenesis of this lesion is not understood (Suttle & Angus, 1976). In sheep the range between copper deficiency and copper toxicity is much smaller than in other animal species. Including of pharmacological dimensions of Cu in pig diets has been a typical practice to improve development performance (Ma et al., 2015). and enhancing Cu to adiets fed to weanling pigs at 100 to 250 mg/kg may decrease post-weaning soucring and improve normal day by day gain and average daily feed intake (ADFI) (Perez et al., 2011). The development advancing impacts of dietary Cu have been credited to its bacteriostatic and bactericidal characteristics(Stahly et al., 1980) on the grounds that Cu may diminish bacterial populaces in the digestive system, which may influence the development and network structure of microorganisms in the cecum and colon (Hojberg et al., 2005).
3.3. Iron
- For fast-growing laboratory and farm animals, especially piglets, iron supply is quite critical during the first few weeks of life when rapid expansion of red-cell mass imposes great demands upon animal and diet to deliver sufficient iron to erythropoietic tissue (Cavill, 2002). Iron deficiency seldom occurs in adult grazing animals. The iron reserves of the newborn calf are usually sufficient to prevent iron deficiency if calves are fed with dry feeds within a few weeks after birth (Moser et al., 1994), but iron supplementation is needed when calves are fed exclusively whole milk (Blum & Hammon, 1999). Iron deficiency anemia begins at 16–20 weeks, when the hemoglobin concentration decreases below 9 g/dl (Welchman et al., 1988), and in order to prevent such an iron deficiency, as well as to optimize the performance and growth rate of calves, an iron supplementation of 100 mg/d has been recommended (Volker & Rotermund, 2000).
3.4. Manganese (Mn)
- Manganese (Mn) is a base metal which can occur principally in 11 oxidation states (–III to +VII), more than any other element. Signs of manganese deficiency in livestock depend on the species. Poultry, and in particular chicks, are most susceptible to dietary manganese deficiency. Perosis is the result of a lowered proteoglycan content of the tibial growth plate, which involves a twisting and bending of the tibia, and slipping of the gastrocnemius tendon from its condyles. In dairy cattle symptoms of manganese deficiency include impaired growth, skeletal abnormalities, impaired reproduction and abnormalities of the newborn. In cattle the manganese requirement for growth is lower than that required for reproduction and birth of normal calves (Karatzias et al., 1995). The manganese recommendation for heifers and dairy cows is 50 mg manganese/kg DM (GfE, 2001). The recommendation for manganese in sheep is 20–40 mg/kg diet (NRC, 1985). For goats 60–80 mg manganese/kg DM are recommended (GfE, 2003).
4. Probiotics
- Direct-fed microbials, which might be all the more usually known as probiotics, are characterized as, "live microorganisms which, when regulated in satisfactory sums, give a medical advantage on the host (FAO/WHO, 2001)." Since 1989, the Food and Drug Administration has necessitated that the term probiotic possibly be utilized when alluding to human microbial items; hence, the expression "DFM" is utilized in the U.S. feed industry, though "probiotic" is utilized reciprocally with human and livestocks feed around the world. The probiotics can be broadly classified into 4 types, for example:a) Bacterial vas Non-bacterial probiotics: with the exception of certain yeast and fungal probiotics (S. cerevisiae), most of the micro-organisms used are bacteria (Mookiah et al., 2014).b) Spore forming versus non-spore forming bacteria as probiotics: in contrast to the previously used probiotics, the modern probiotics are based on the spore forming bacteria which sustain comparatively longer in animal system (Ahmed et al., 2014).c) Multi-species (multi-strain) probiotics versus Single-species (single-strain) probiotics.d) Allochthonous probiotics versus Autochthonous probiotics: micro-organisms which are normally not present in GIT of animals (allochthonous) includes yeasts, while the micro-organisms normally present as indigenous inhabitants of GIT (autochthonous) include Lactobacillus and Bifid bacterium.To minimize the gaseous losses during the fermentation process and therefore, improving the efficiency of feed utilization and overall performance of the dairy animals, manipulation of rumen is being given more and more emphasis by the ruminant nutritionists. For this purpose, probiotics like, Yeast has commonly been used (Chaucheyras-Durand et al., 2008), which effects the microbial population dynamics in rumen and breakdown of nutrients. The improved performance of animals due to probiotics application is often due to the improved digestibility. Bacterial population in the silage is also a good source of probiotics to the ruminants (Weinberg et al., 2004). Boyd et al. (2011) used a combination of L. acidophilus and P. freudenreichii and found that digestibility of crude protein and fiber improved significantly in Holstein cows. Nourishing DFM has been recommended to increment cellulolytic microbes in the rumen of dairy animals (Dawson et al., 1990) and to upgrade dietary fiber fermentation in the horse (Godbee, 1983). The meta-analysis of the application of yeast probiotics in ruminants by Desnoyers et al. (2009) demonstrated that live yeast significantly increased rumen SCFA and increased rumen PH. Higher the proportion of concentrate and neutral detergent fiber in the diet, the better is the digestibility of organic matter resulting from the live yeast supplementation. The increased cellulose degradation and microbial protein production due to yeast-based probiotics in ruminants is mainly due to the increased number of the cellulolytic bacteria (Dawson et al., 1999). Similarly, increase in the number of rumen bacteria in cross bred cattle fed S. cerevisiae probiotic has been reported by Ding et al. (2014).Feeding with high non-structural carbohydrates e.g. starch and low fiber diet lowers the ruminal PH (Duffield et al., 2004) leading to accumulation of SCFAs and unbalanced buffering of rumen (Plaizier et al., 2008). The condition is referred to as SARA when the pH drops below 5.6 (Gozho et al., 2005) leading to loss of appetite, diarrhea, dehydration, debilitation, impaired rumen motility and impaired fiber digestibility. Lactic acidosis is the more severe form of ruminal acidosis where the PH drops below 5.2 due to accumulation of lactate (Owens et al., 1998). Including of DFM to swine diets may improve intestine wellbeing by changing the micro flora, which may help control pathogens (Prescott et al., 2005), upgrade immune regulation and reaction (Galdeano and Perdigon, 2006), increment nutrient digestibility (Giang et al., 2011), improve wellbeing status, and improve pig development execution (Kenny et al., 2011; Cromwell, 2013). Addition of DFM to diets may likewise decrease the immune incitement, shown by a decrease in pro-inflammatory cytokines in enterocytes, which may move vitality typically utilized for excessive immune incitement to development, in this manner improving feed productivity (Cho et al., 2011).Probiotics have also been found to effective in alleviating the acidosis. Lettatet al., (2012) studied the effect of application of Propionibacterium, L. plantarum and L. rhamnosusstrain as probiotics and found them to be effective in pH stabilizing and prevention of acidosis in sheep. It was hypothesized that stability in ruminal pH was achieved by the probiotics modulating rumen microbes so that their capacity to hydrolyse cellulose was increased and lactic-acid producing bacteria were inhibited.Yeast might be supplemented in diets fed to pigs in a few structures: entire live yeast cells, heat-treated yeast cells, ground yeast cells, cleansed yeast cell cultures, and yeast extracts. Live yeast cells can possibly play a double probiotic– prebiotic dynamic job in the rumen. The probiotic activity of the yeast can be clarified through regulating the sythesis and activities of the rumen microbial biological system, prompting a lessening in lactic acid substance, an expansion and support of ruminal pH, an improvement in nutrient absorbability, an advancement of VFA profiles, and a decline in ruminal ammonia creation (Wang et al., 2016). Moreover, direct-fed yeast cells inspire a prebiotic impact through mixes, for example, oligosaccharides, amino acids, vitamins, and organic acids contained inside yeast cells, all of which can invigorate microbial networks in the rumen to develop (Opsi et al., 2012). In this manner, dietary yeast can beneficially affect ruminal effectiveness. Yeast or yeast-based item supplementation may support ADFI and growth performance, increase mucosal immunity, advance gut development, adsorb myco-poisons, lessen post-weaning diarrhea, and modulate gut micro biota (Sauer et al., 2011; Jiang et al., 2015).
5. Prebiotics
- Prebiotics are chiefly non digestible oligosaccharides and have been characterized as "non-digestible food ingredients that advantageously influences the host by specifically invigorating the development and additionally action of one or a predetermined number of microscopic organisms in the colon, and consequently improves host wellbeing” (Gibson & Roberfroid, 1995). Schrezenmeir & De-Vrese (2001) classify them as pharmaceutical grade nutrients. They contain nutrients that stimulate growth of beneficial intestinal micro flora in the animal's digestive tract and suppress harmful pathogenic bacteria from the body (Semeniuk et al., 2008). Inulin, fructo-oligosaccharides, transgalacto-oligosaccharides, and lactulose are the most well-known carbohydrates that have been perceived as prebiotics in light of the fact that these carbohydrates are effectively fermentable and accordingly will result in diminished luminal pH (de Lange et al., 2010; Bach Knudsen et al., 2012). Be that as it may, other dietary carbohydrates, for example, arabino-xylans, xyloglucans, and resistant starch likewise may have prebiotic impacts (Bach Knudsen et al., 2012). Prebiotics additionally might be gotten by cchemical processing that hydrolyze polysaccharides (i.e., isomalto-oligosaccharides from starch) or by enzymatic or chemical synthesis from disaccharides (Broek et al., 2008). Most prebiotics are synthesized or detached from plant and algae polysaccharides (Saad et al., 2013; Wu et al., 2017).According to Grela et al. (2013) addition of prebiotics to dairy animal feed has inhibited the development of pathogenic microorganisms in the digestive tract with a decrease in the population of E. coli that are the main pathogens causing diarrhea in animals. These substances are produced by surface yeast cell walls. Prebiotics increase the microbial diversity in the host GIT leading to improved feed utilization (Krause et al., 2010). In ruminants, the presence of a huge, dense pre-gastric microbial population in the rumen break down many of prebiotic compounds and presents enormous challenges to the implementation of prebiotics in these animals. Another factor working against prebiotic usage in ruminants is the large GIT volume (Russell, 2002). This has limited the number of investigations regarding the use of prebiotics in ruminants; however, rumen-protective technologies may allow these compounds to be used in dairy animals (Baines et al., 2011).
6. Synbiotics
- Simultaneous use of probiotics and prebiotics together is known as “synbiotics”. These two products support each other in a highly targeted fashion, which has been reported the most likely approach to reduce pathogens in dairy animals (Vandeplaset al., 2010). Bombaet al. (2002) showed a synergistic effect in reduction of food borne pathogenic bacteria populations in food animals when fed synbiotics.
7. Ionophores
- Ionophores are organic compounds mainly from Streptomyces spp. that facilitate selective transportation of ions across the outer cell membrane. In spite of their unique aim as a coccidostat, ionophore antibiotics have been seemingly the best feed added substance utilized in ruminant production for a long time. Despite the fact that nourishing ionophores has appeared to be effective in numerous production settings, their utilization is particularly across the board in the meat feedlot industry. Be that as it may, ionophores remain underused in grazing conditions. Ionophores affirmed for use in livestock production incorporate Monensin Sodium, LasalocidSodium, Salinomycin, and Laidlomycin Propionate Potassium (Russell, 2002). The response of nourishing ionophores to ruminants is well-archived and shifts somewhat relying upon the compound. Generally speaking, ionophores decline feed consumption without decreasing development to improve feed conversion. The feed proficiency benefits come about because of expanding energy and nitrogen digestion and ruining the event of digestive disorders (Bergen & Bates, 1984).monensin is mostly used ionophore in the dairy animals. It is recommended orally as monensin sodium (Hobson & Stewart, 2007). By reducing the fiber digesting gram positive bacterial and increasing the concentrate utilizing gram negative bacteria, the ionophores change the pattern of rumen fermentation towards higher propionate production and decreased methanogenesis. Gram-positive bacteria lack the complex cell wall of gram negative bacteria and the associated lip polysaccharide layer with its protein channels (porins) that have a size exclusion limit (600 Da) that is impervious to ionophores (> 600Da) as suggested by McGuffey et al. (2001) (McGuffey et al., 2001). Consequently, ionophores successfully infiltrate the outer cell membrane of gram-positive bacteria and rapidly and repeatedly cause efflux of intracellular K+ from the cell and influx of extracellular protons (Na+ and H+). The resulting cytoplasmic acidity causes cell death of gram positive bacteria (Guffanti et al., 1979). Application of ionophores in ruminant diet leads to increased propionate while decreased acetate ad methane production resulting in enhanced feed utilization efficiency of the dairy animals. Monensin has a benefit to cost ratio of 5 to 1 when added to dairy cow diets; it is recommended for increasing feed efficiency in lactating cows and reducing metabolic disorders in dry cows (Mecitoglu et al., 2017). Ionophores are also labeled as a coccidiostats in growing heifers leading to improved growth and health. These are recommended at dose rate of 300 to 350 milligrams per cow per day. In spite of the fact that the efficacy of ionophores has been illustrated, their order as an antibiotic may put their future use in danger regardless of no relevance to human medicine.
8. Nucleotides
- Dietary nucleotide supplementation has been related with both humoral and cell immunity, yet the detailed mechanismhas not been illustrated. Dietary nucleotides add to the flowing pool of nucleosides accessible to invigorate leukocyte creation (Kulkarni et al., 1994; Carver and Walker, 1995). Hence, there is a raised requirement for nucleotides amid times of immunological difficulties. Supplementation of eating regimens with nucleotides may expand the quantity of lymphocytes and macrophages of the intra-epithelium of the piglet ileum (Domeneghini et al., 2004; Speranda et al., 2008), decline harm of the blood lymphocyte DNA (Salobir et al., 2005), decline the concentration of TNF-a and IL-6 in blood serum 2 and 4 h after an E. coli infusion (Hung, 2015), and increment plasma and serum concentration of IgA (Sauer et al., 2012a, b) in pigs. Dietary nucleotides improve intestinal retention of iron, influence lipoprotein and long chain polyunsaturated unsaturated fatty acid digestion, effectsly affect the intestinal mucosa and liver, and lessen the occurrence of diarrhea (Schlimme et al., 2000).
9. Phytogenic Feed Additives
- A gathering of added substances that got a dynamic enthusiasm for as far back as years comprises of plant secondary metabolites (PSM) and plant-derived extricates which are in charge of the scent and shade of plants (Patra and Saxena, 2010). Plant extricates are made out of in excess of a hundred individual parts and in two unique structures: fluid oil and strong powder. A large portion of the oil framed plant extricates are water-insoluble and regularly called essential oils. Plant extracts might be separated from plants through steam refining, maceration, cold squeezing, and solvent extraction or might be synthesized (Kerrola, 1995). Nutritionists have for quite a while considered plant secondary metabolites (tannins, flavonoids, and saponnins) as anti-nutritional components attributable to their impeding impacts on feed admission, nutrient use, livestock profitability, and wellbeing (Sarwar et al., 2012). Notwithstanding, ongoing exploration has demonstrated that the negative or positive outcomes of secondary metabolites rely upon their origin, chemical properties, dietary concentration, and feed quality. In this manner, discoveries acquired from various in vivo and in vitro examinations with dairy cattle, sheep, and goats suggest joining restricted measures of secondary metabolites into food to improve feed use.Plant extracts are of potential enthusiasm because of their potential natural capacities, for example, antiviral, antimicrobial, antioxidant, and anti-inflammatory impacts (Liu et al., 2014). This may prompt the capacity to utilize plant extracts to supplant antibiotics in-feed to improve execution and soundness of livestocks (Pettigrew, 2006; Stein & Kil, 2006). These mixes are expected to positively affect livestock groth by improving ruminal fermentation, moving the ruminal microbial environment to contain less methane-delivering microorganisms, decreasing inordinate dietary protein corruption, and actuating microbial protein generation (Cobellis & Trabalza-Marinucci, 2016; Wanapat et al 2012). In light of the literature, plant concentrates may improve livestocks wellbeing through a few systems, for example, direct concealment of the expansion of pathogens, change of gut microbial populaces, and upgrade of immune capacities. Anti-microbial activities of different plant extricates have been well organized. Plant extracts display a wide range of antibacterial exercises against gram-negative and gram-positive microscopic organisms, including Escherichia, Salmonella, Staphylococcus, Klebsiella, Proteus, Bacillus, Clostridium, and Mycobacterium (Dorman & Deans, 2000; Wong et al., 2008).A scope of essential oils (EOs) (Calsamiglia et al., 2007) was accounted for to diminish methane generation in vitro. Some accomplishment with other plant extracts, specifically those wealthy in tannins (Grainger et al., 2009), saponins (Wang et al., 2009) and flavonoids (Broudiscou et al., 2000), was depicted, as well. Notwithstanding their modulatory impact on rumen microbial populace, PSM appear to be a fascinating gathering of feed added substances because of the positive effect on nourishing stress, including bloat and acidosis, and generally speaking livestock' wellbeing and profitability (Acamovic & Brooker, 2005; Rochfort et al., 2008). Also, in poultry and swine phytogenic items were seen to lessen bacterial colony counts, reduce gut fermentation, decline the movement of the gut-related lymphatic system and advance intestinal mucus generation (Hashemi & Davoodi, 2010).
9.1. Essential Oils
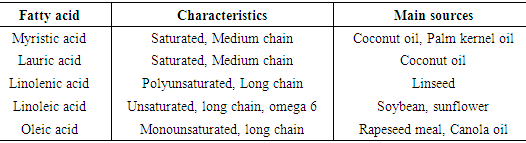
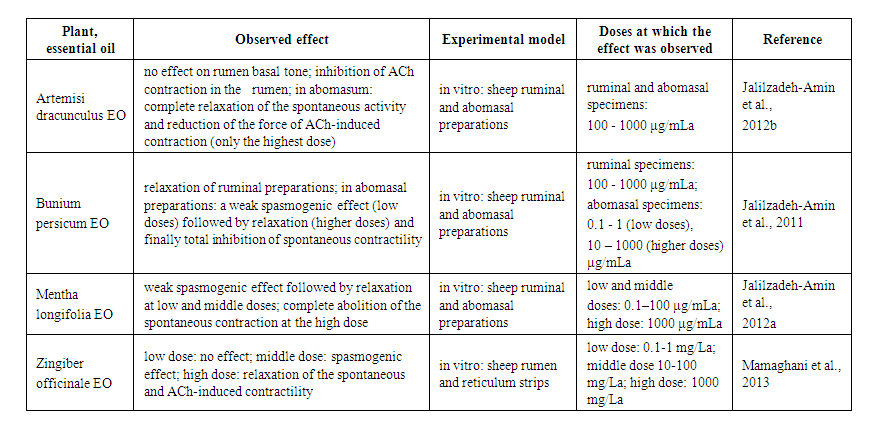
- Essential oils (EOs) are volatile, complex mixes portrayed by a strong scent. They are removed from different sweet-smelling plants commonly situated in mild to warm nations as the Mediterranean and tropical nations. Within the category 'Essential oils', 42 substances were covered by the EFSA review and 31 were found to offer potential for reducing ruminal methane and ammonia, in many cases reduction in these pollutants occurred simultaneously (Lewis et al., 2013). The most promising essential oil with over 5 studies, that included in vivo work, was Allium arenarium oil (garlic oil) which reduced methane by 12% in vivo and an average of 36% reduction was reported in vitro (Lewis et al., 2013). Essential oils are being joined into livestock feeding for three noteworthy reasons: to improve feed qualities because of their anti-oxidative and antimicrobial characteristics; to upgrade absorption and general execution; and, to improve attributes of livestock products (Franz et al., 2010). The anti-oxidative activity of EOs adds to the insurance of feed lipids from oxidative harm upgrading the nature of ingested feed. Additionally, in small ruminants the long-term admission of EOs was found to improve antioxidant stability of the meat and milk and to bring down powerlessness of oxidative stress in suckling goats' kids (Martinez et al., 2007). The consequences of in vitro investigations demonstrate their conceivable use as rumen modifiers (Castillejos et al., 2008). The impacts of individual essential oils and plant extricates wealthy in EOs are outlined in Tab.1.
 | Table 1. The effect of plant essential oils (EO) |
|
9.2. Tannins
- Tannins are water dissolvable phyto-mixes of different atomic loads, variable multifaceted nature and contain countless phenolic hydroxylic bunches which empower them to frame viable cross-joins with proteins and to a lesser degree with metal particles, amino acids and polysaccharides (Kamra et al., 2008; Rochfort et al., 2008). Tannins are a standout amongst the most broadly examined secondary plant metabolites in livestock Nourishment (Costa et al., 2017). These mixes are fit for shielding dietary proteins from fast and exorbitant debasement in the rumen by shaping insoluble tannin– protein buildings, which decrease dietary protein breakdown (Lorenz et al., 2014). Subsequently, less ammonia is delivered, nitrogen misfortune from dietary protein diminishes, and the progression of amino acids to the small digestive tract expands (Mokhtarpour et al., 2016). In this way, supplemental tannins can upgrade the productivity of dietary protein use by expanding the progression of amino acids to the small digestive tract.The decrease of protein corruption in the rumen when tannins are supplemented to livestock feeds happens because of diminishing ruminal proteolytic bacterial populaces, for example, Clostridium proteoclasticum, Butyrivibrio fibrisolvens, Eubacterium sp., and Streptococcus bovis (Patra and Saxena, 2011; Anantasook et al., 2013). Tannins could moderate methane emanations by diminishing the development of transcendent methanogenic microscopic organisms, protozoa, and archaea (Yang et al., 2017). Such an impact would decrease energy losses and permit progressively accessible energy and protein for livestock use. What's more, the incitement of ruminal biohydrogenation of polyunsaturated atty acids saw when tannins are supplemented in vitro creates more noteworthy measures of trans 11-18:1 and cis 9, trans 11-18:2 unsaturated fatty acids, the two of which are viewed as valuable for human wellbeing (Costa et al., 2017). Consequently, the job of tannins in ruminant eating diets goes past ruminal protein digestion.Generally, the positive outcome of dietary tannin supplementation is through direct effects on rumen organisms and their digestion. Dietary tannins can prevent swelling, which is a serious digestive disorder that happens when dietary proteins are debased quickly in the rumen, framing a steady foam that traps ruminal gases, represses eructation, causes serious agony, diminishes prod uction efficiency, and may result in animal death (Patra and Saxena, 2011; Wang et al., 2012). Owing to the effectiveness of tannins in framing tannin– protein buildings to lessen the unnecessary ruminal protein corruption and abatement methane generation, supplemental tannins assume a key job in controlling the rate of bloat in ruminants.
9.3. Saponins
- Saponins are sugar and non-carbohydrate complexes that characteristically foam when shaken in water. They are steroid or triterpene glycoside compounds found in a variety of plants. They are generally disseminated in higher plants, and somewhat in lower marine creatures and in certain microscopic organisms (Osbourn, 1996). Their surface-dynamic properties are what recognize these mixes from different glycosides. The diet of ruminants incorporates a few plants wealthy in saponins, prevalently alfalfa and clovers that are an important wellspring of protein for temperate climates (Cheeke, 1971).The developing enthusiasm for the utilization of saponins as phytogenic feed added substances results from their various natural effects, for example immuno-stimulatory, anti-inflammatory, hepato-defensive, antibacterial, antiviral, antifungal and against parasitic properties (Francis et al., 2002; Sprag et al., 2004).In vitro and in vivo experiments show how saponins can be used to remove or reduce protozoa numbers in the rumen (described in a review by Wina et al. (2005)). As of late, there have been adequate in vitro and in vivo proof of methane creation hindering action (Flachowsky and Lebzien, 2012; Patra and Yu, 2013), against protozoal impacts of saponins (Sanosto et al., 2007) and their capacity to adjust rumen fermentation by means of direct effect on the arrangement and action of rumen protozoa, bacteria, fungi and archaea (Patra & Saxena, 2009). A review of studies by Wina et al. (2005) indicated that improvements in live weight gain are most likely to be seen with saponin addition to high roughage diets, although this was not clear cut, some moderate to high roughage diets still showed no growth enhancing effect (Hussain & Cheeke, 1995). Also, sex can interplay with the response: a higher growth response was reported in male lambs compared to female’s lambs with 40 mg quillaja saponins/kg in low roughage diets (Hussain & Cheeke, 1995).Commercial additives are derived from Yucca shidigera or Quillaya saponaria and Sapindus sp (temperate and tropical plant species). Their extracts are as of now utilized as dietary added substances for domesticated and companion animals. Notwithstanding the broad use as methane generation modifiers saponins are considered as powerful enemy of parasitic agents (Mehlhorn et al., 2011). The main issue with feeding saponins to ruminants is evidence that the rumen microbe adapt and protozoa return to pre-supplementation levels (Newbold et al., 1997), intermittent additions may mitigate this effect (Thalib et al., 1996). There is also evidence that they can be degraded in the rumen (Wang et al., 2000).
9.4. Sensory Additives
- Traditional sensory additives include substances affecting food odor and palatability, and colorings. Phytogenic additives are commonly used as colorings in laying hens to affect the egg yolk color (Englmaierova et al., 2014). Natural colorings are preferred; the most frequently used ones include carotenoids, the source of which are carrot, Chlorella algae, marigold (Tagetes erecta L.), or lutein, however, natural carotenoids are unstable and their use is also limited by their price (Englmaierova et al., 2014). Well known carotenoids include also astaxanthin, which is commonly added to fish feed to provide for a more attractive meat color. Besides, this substance also has strong antioxidant effects (10× more effective than vitamin E); it is also a subject of recent studies for its effect against reactive oxygen species (ROS) (Gomez et al., 2013), or for its neuroprotective effect in subarachnoid haemorrhage (Zhang et al., 2014).
9.5. Immunomodulators
- One of the main aims of using phytogenic additives is their potential effect on the immune system. Important immunomodulators include oligosacharides, in particular β-glucans. These substances are obtained mainly from fungi, Basidiomycetes and yeast of the genus Saccharomyces ceverisiae (Wojcik, 2014a); their effects are studied in a number of animal species, e.g. in cattle (Wojcik, 2014a, b), sheep (Milewski et al., 2013; Zabek et al., 2013) or pigs (Sorocinova et al., 2013). Beta-glucans can be found in plant components, too, traditionally in the aleuronic layer of barley and oat bran, or seaweed. Extraction of β-glucans from plant components is demanding not only financially (El Khoury et al., 2012); nevertheless, bamboo leaf extracts appear to be a new and promising source of β-glucans. Ohtsuka et al. (2014) evaluated the effect of an extract of β-glucans obtained from bamboo leaves (Sasa sensanensis) in cattle. The authors found mainly higher activity of CD8+ T lymphocytes, i.e. cells that are crucial for the immune response to a number of viral infections.Essential oils that exhibit antimicrobial properties and at the same time have minimum impact on the health of the host organism (Rada et al., 2009). Essential oils are used in a number of animal species, among others, also fish and honey bees. Phytogenic feed additives (PFA) containing essential oils of thyme and star anise as main active components were studied by Cho et al. (2014) who examined their effect on the growth performance, energy, nutrient apparent total tract digestibility, blood metabolites, intestinal micro flora, meat color, and relative organ weight after oral challenge with Clostridium perfringens in broilers. They found improved growth performance, reduced blood total cholesterol, and also inhibited C. perfringens and E. coli proliferation in small and large intestines in the group of broiler chicks with the addition of PFA compared to the control group and the group with the addition of antibiotics after application of Clostridium perfringens. The anti-oxidative effect of evening primrose oil (EPO) administration (150 ml) on the oxidative stress of race horses during their regular training period was determined in the study by Mikesova et al. (2014).
10. Enzymes as Feed Additives
- These are natural biocatalysts which regulate different biochemical reaction in the living system. They can also be employed as feed additives for improving the deragation reaction during feed digestion. Numerous enzyme supplements are used to increase the digestibility of non-starch polysaccharides of whole grains, particularly in non-ruminant food animals like chickens and swine, and thus improve their feed utilization and act as growth promoters. The enzymes produced by the rumen microbes work in a synchronous fashion for carbohydrate digestion. The capacity to solubilize in ruminal fluid, structural complexity and accessibility to the rumen microbes are the main factors that determine the efficiency of fermentation of the carbohydrates in rumen (Nagaraja et al., 1997). The ruminant nutritionists have started giving more emphasis on manipulating the rumen carbohydrate and protein digestion metabolism to maximize the efficiency of degradation of feed. Cellulases, xylanases, β-glucanases, pectinases, amylases, proteases, phytases and enzymes that degrade specific plant toxins like tannases,arise from the diversity of the microbial population established in the rumen (Kumar et al., 2018).Exogenous fibrolytic enzymes like cellulases or xylanases have been extensively used in non-ruminant animals since long. But, their use in ruminants has been started over 40 years ago only (Rust et al., 1965). Xylan is a linear polysaccharide which is a component of hemicellulose, one of the major building block of plant cell walls. Xylanase is the name of a family of enzymes which degrade xylan into a simple sugar called xylose, thus breaking down hemicellulose. Mammals do not produce xylanase but the enzyme is commonly found in microbes where it causes degradation of plant matter into usable nutrients. Xylanases are commonly used as feed additives, for example in poultry, helping extract nutrients from grains and vegetable feed with a high fiber content. Recent patents describe xylanases and encoding nucleic acids (Weiner et al., 2011) and thermo-stable xylases which are suitable as feed additives, particularly for poultry and swine (Sung & Tolan, 2011). Exogenous enzymes in ruminants alter feed utilization either through their effects on the feed before ingestion, or through improvement in the digestion in rumen or post-ruminally.Phytate is an inositol hexakisphosphate and is the main storage form of phosphorus in many plant tissues. Inositol and phosphorus in phytate are bioavailable to ruminants due to digestion by the enzyme phytase produced by rumen microorganisms. In contrast, phytate is indigestible to non-ruminant animals because they naturally lack the digestive enzyme. In non-ruminant livestock, such as poultry and swine which are fed mainly grains and soybeans, the undigested phytate is excreted in the manure. In these farm animals, phytate inositol and phosphorus can be made available by adding phytase to their feed. New technologies, such as recombinant DNA technology, have made phytase more practical and economical as feed additive (Cromwell, 2008).Enzymes with lipase activity are also used as feed additives. A US patent application (Cromwell, 2008) describes polynucleotides encoding polypeptides with a hydrolase activity. The hydrolases, including lipases, can be used as feed supplement, optionally to hydrolyze long chain fatty acids. Another US patent application (Liu, 2012) discloses a polypeptide having pancreatic lipase activity and the nucleic acid encoding it. The polypeptide is particularly suited for preparation of feeds for post-weaning pigs to enhance their growth performance. Different feed types, application levels of enzymes, type of enzyme products and enzyme application methods are the different factors which effect the response from the animals and have been compared under controlled conditions with better feed efficiency outcome (Kumar et al., 2018). Although it was demonstrated that these enzyme preparations could increase milk production in cows fed total mixed rations, positive responses in milk production were highly dependent on the level of enzyme applied (Sanchez et al., 1996). Beaucheminet al. (1998) suggested that the efficacy of enzymes in ruminant ration is apparently dependent upon the method of its application.
11. Electron Receptors
- Electron receptors such as nitrate and sulphate were not considered in the EFSA review, yet are prominent in other recent reviews on methane mitigation (Hristov et al., 2013; Hristov et al., 2013). They offer an alternative a hydrogen sink to carbon dioxide, thereby reducing methane emissions and potentially improving digestion efficiency (Ungerfeld & Kohn, 2006). A comprehensive review suggests that sheep can tolerate up to 4% nitrate in high quality concentrate or forage based diets without showing clinical signs of methamoglobinemia (Leng, 2008). Although overall, potassium nitrate addition did reduce methane emissions in the study by Nolan et al. (2010) after the immediate reduction observed post feeding, there was a gradual rise in methane over the two hours between feeding periods. The authors suggest a ‘slow release’ form of nitrate might enhance methane mitigation and, in addition, reduce nitrate and nitrite absorption from the rumen and thereby reduce the potential for nitrite toxicity.
12. Conclusions
- The restrictions on the use of growth promotants, including hormonal implants and beta-agonists, has increased the attention and research to improve growth performance via rumen function optimization and regulating intestinal environments to recover production losses for elimination of older, more proven technologies. Several feed additives may be effective in improving livestock growth performance. For example, acidifiers are often used as alternatives to antibiotic growth promoters because of their ability to create a favorable intestinal environment for beneficial microbes which may result in increased nutrient digestibility, increased growth performance, and reduced diarrhea. Essential trace elements (e.g. Fe, Zn, Mn, Cu, I, Se, Co, Cr, Mo, Ni) are the most important inorganic feed additives in the nutrition of livestock. Their function as integral parts of diverse proteins, enzymes and hormones involved in fundamental metabolic and protective (anti-oxidative) processes make them indispensable. Feeding DFM has been recommended to increment cellulolytic microscopic organisms in the rumen of cows and to improve dietary fiber fermentation in horses. Prebiotics gainfully influences the host by specifically invigorating the development as well as activity of one or a predetermined number of microscopic organisms in the colon, and in this manner improves host wellbeing and development performance. have been apparently the best feed added substance utilized in ruminant generation for a long time and can diminish feed intake without decreasing development to improve feed conversion. The feed effectiveness benefits come about because of expanding energy and nitrogen digestion and ruining the event of stomach related disorders. Supplementation of eating regimens with nucleotides may upgrade intestinal assimilation of iron, influence lipoprotein and long chain polyunsaturated fatty acid digestion, effectsly affect the intestinal mucosa and liver, and improve the immune reaction. The growing interest in the use of phytogenic feed additives results from their numerous biological activities, e.g. immune-stimulatory, anti-inflammatory, hepato-protective, antibacterial, antiviral, antifungal and anti-parasitic properties. This may prompt the capacity to utilize plant extracts to supplant antibiotics in-feed to improve execution and soundness of livestock. Exogenous fibrolytic enzymes like cellulases or xylanases have been extensively used in non-ruminant animals since long but it was suggested that the efficacy of enzymes in ruminant ration is apparently dependent upon the method of its application. There are various feed added substances that conceivably might be utilized, yet the fundamental challenge with these added substances is the way that outcomes acquired so far have been conflicting. The explanation behind this irregularity might be that efficiencies of every added substance are diet dependent and furthermore subject to the wellbeing status of the livestock. Generally, the most of these additives are considered safe for addition in feed, nevertheless their toxicity towards animals must be carefully verified, especially in regard to newer substances.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML