-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(6): 213-219
doi:10.5923/j.ijaf.20180806.03

Performance and Blood Profile of Growing Pullets Fed Diets Supplemented with Cholecalciferol
Adedeji B. S.1, Ogunwole O. A.1, Olumide M. D.2, A. O. Mosuro1
1Agricultural Biochemistry and Nutrition Unit, Department of Animal Science, University of Ibadan, Nigeria
2Department of Animal Science, School of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Ogun State, Nigeria
Correspondence to: Adedeji B. S., Agricultural Biochemistry and Nutrition Unit, Department of Animal Science, University of Ibadan, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

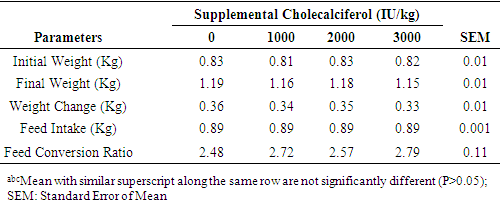
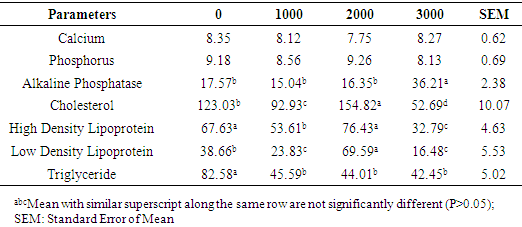
Nutritional additives in animal production are often targeted at further improving productivity. Supplementation beyond the conventional requirement of animals however, may make or mar animals’ performance and well being. This study was conducted to assess the effects of dietary supplemental cholecalciferol on performance and blood profile of growing pullets. Bovan Brown pullets (n=128) aged 12 weeks and weighing 0.82±0.03 were allotted to four dietary treatments of four replicates and eight pullets per replicate in a completely randomised design. They were initially raised on an iso-caloric and iso-nitrogenous basal diet for the first three weeks and thereafter the diets were supplemented with 0 (T1), 1000 (T2), 2000 (T3) and 3000 IU/Kg D3 (T4), respectively. At week 20, final weight (kg) (1.19, 1.16, 1.18 and 1.15), weight changes (kg) (0.36, 0.34, 0.35 and 0.33), feed intake (0.89, 0.89, 0.89 and 0.89) and feed conversion ratio (2.48, 2.72, 2.57 and 2.79) for pullets on T1, T2, T3 and T4, respectively were not significantly affected (P>0.05) by the treatments. Platelet was the only haematological parameter that was significantly increased (P<0.05) by vitamin D3 supplementation. Platelets of pullets on 1000 IU D3 (2.36) was significantly higher (P<0.05) than 1.55, 1.51 and 1.63 in pullets on 0, 2000 and 3000 IU D3. Serum calcium and phosphorus were not significantly (P>0.05) affected by dietary D3 supplementation. The ALP, however was significantly higher (P<0.05) at 3000 IU supplementation (36.21) compared to similar (P>0.05) values of 17.57, 15.04 and 16.35 in pullets on 0, 1000 and 2000 IU D3 supplementations, respectively. The highest serum cholesterol (154.82), HDL (76.43) and LDL (69.59) levels were observed in pullets on supplemental 2000 IU D3 compared to other treatments while triglyceride was lowered (P<0.05) with increasing level of D3 supplementation from 82.58 in T1 to 45.59, 44.01 and 42.45 in those on 1000, 2000 and 3000 IU D3, respectively. Supplemental vitamin D3 in the diets of pullets had no gross effect on performance attributes and blood indices monitored.
Keywords: Growing pullets, Supplemental cholecalciferol, Dietary vitamin, Serum cholesterol
Cite this paper: Adedeji B. S., Ogunwole O. A., Olumide M. D., A. O. Mosuro, Performance and Blood Profile of Growing Pullets Fed Diets Supplemented with Cholecalciferol, International Journal of Agriculture and Forestry, Vol. 8 No. 6, 2018, pp. 213-219. doi: 10.5923/j.ijaf.20180806.03.
Article Outline
1. Introduction
- Nutritional additives are becoming a normal pre-requisite in the formulation of animal feeds as their inclusion in diets is associated with increased livestock production. Supplemental vitamins are among such additives of immense importance in livestock production. Vitamin requirements established decades ago do not take into account the modern genetically superior birds with increased growth, egg production and improved feed efficiency. Vitamin intake per unit of output is continually declining, as yearly decline for layers is around 1% per egg produced, while for broilers is 0.6-0.8% for body gain [1]. Cholecalciferol also known as vitamin D3 plays a major role in livestock production because of its involvement in the maintenance of skeletal integrity. It can be synthesized in the skin, catalyzed by ultraviolet radiation, from 7-dihydrocholesterol present in the dermis and epidermis [2] or be supplied in the feed. Commercial chickens are usually maintained indoors, and do not receive enough solar radiation to convert 7-dihydrocholesterol in sufficient levels to supply their cholecalciferol requirements. This is importantly the reason why cholecalciferol is routinely added to poultry feed for the maintenance of egg production, eggshell formation and calcium homeostasis. After absorption of D3 by the intestinal mucosa, it is transported to the liver, where it is hydroxylated in the carbon position 25, resulting in 25-hydroxycholecalciferol (25(OH) D3). This metabolite is thereafter hydroxylated at carbon 1, in the kidney thus resulting in the active metabolite 1,25 dihydroxycholecalciferol (1, 25(OH)2D3) [3]. This active form stimulates bone calcium mobilization and inhibits urinary calcium excretion when hens are calcium deficient or need more calcium, through molecular mechanisms involving bone cells [4], vitamin D binding proteins [5] such as calbindin [6, 7] and ovocleidin-17 [8] as well as other hormones such as estrogen and thyroxin [9]. Most documentation on D3 are associated with calcium homoestasis with avalanche of information in broiler and laying hens but little information in growing pullets. Also studies in poultry had hitherto laid emphases on egg production and bone integrity [10-16] with very little attention drawn to the effect on performance attributes and blood profile of chickens. Blood is an important index of physiological, pathological and nutritional status in animals [17, 18]. Aletor [19] and Egberongbe [20] observed that blood variables were most constantly affected by dietary factors. Since supplements were often added above the NRC [21] recommended dosage in feed, the safety of such additional supply in feed needed to be assessed in the blood. Also, the appropriate supplemental dosage in chicken’s feed that would exert no detrimental consequence on productivity needs to be documented. The study was therefore aimed at investigating the effect of supplemental cholecalciferol on performance attributes and blood profile of growing pullets.
2. Materials and Methods
2.1. Experimental Site
- The study was carried out at the poultry unit of the Teaching and Research farm, University of Ibadan, Ibadan, Nigeria. The university is located in Ibadan in the tropical rain forest zone of Nigeria within latitude 7° 26 N and longitude 3° 54 E, with a mean altitude of 277 meters above sea level. Average temperature and relative humidity of the location is about 26.5°C and 55%, respectively.
2.2. Experimental Animals and Management
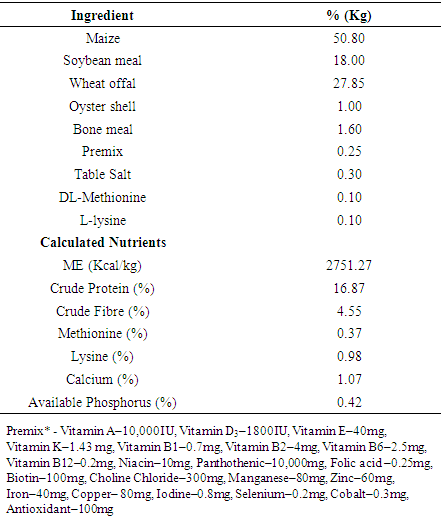
- Bovan Brown pullets (n=128) aged 12 weeks and weighing 0.82±0.03 were purchased from a reputable farm and allotted to four dietary treatments of four replicates and eight pullets per replicate in a completely randomised design. Pullets were housed in a conventional 3-tier battery cage housing system. Each cubicle measured 50 x 45 x 40 cm3 with a floor space of 450cm2/ bird that accommodated four pullets. Pullets were initially raised on an iso-caloric and iso-nitrogenous basal diet for the first three weeks after which the diets were supplemented with 0 (T1), 1000 (T2), 2000 (T3) and 3000 IU/Kg D3 (T4), respectively.Details of the basal experimental diet have been previously documented and are shown in Table 1 [22, 23]. Routine management practices including vaccination and drug administration were adhered to while feed and water were offered to the pullets ad libitum. The duration of the experiment was eight weeks.
|
2.3. Experimental Design
- The experiment was a completely randomized design and the experimental model is as follows:Yij = μ+ αi + eijWhere Yij = j-th observation of the ith treatment μ = Overall population meanαi = Effect of ith level of cholecalciferol supplementationeij = Random error assumed to be independently and normally distributed with zero mean and variance σ2.
2.4. Data Collection
2.4.1. Performance Characteristics
- The pullets were weighed at the inception of the trial and then subsequently on weekly basis. The pullets in each replicate were weighed individually and weight gain (kg) was calculated on a weekly basis by deducting the initial weight from the final weight. Feed intake was recorded weekly by subtracting the left over from the total feed served. Feed Conversion Ratio (FCR) was obtained by dividing feed intake (kg) with weight gain (kg).
2.4.2. Blood Collection and Evaluation
- At week 20, blood (5 mLs) was sampled from three pullets per replicate using needles and syringes from the jugular vein into heparinised and non heparinised bottles for haematology and serum analyses, respectively. Haematological parameters assessed were white blood cells (WBC), platelets, leukocyte differential count: lymphocytes, heterophils, monophils, eosinophils and basophils [24, 25] while blood samples for serum analysis were left to clot and the serum was separated immediately by centrifugation at 3500rpm for 10 minutes. The serum biochemical parameters assessed were alkaline phosphatase [26], Serum Ca and P [27]. The serum lipids examined were triglycerides (Trinder's enzymic method), total cholesterol [28] cholesterol profile (High density lipoprotein and low density lipoprotein) according to Friedwal et al. [29] and [30]. Samples were read using spectrophotometry at wave length specific for each parameter.
2.5. Statistical Analysis
- Data were subjected to One-way ANOVA using General Linear Model of SAS [31] and means separated using NDMRT option of the software at α0.05.
3. Results
- Performance of point of lay pullets (POL) fed diets supplemented with varying levels of cholecalciferol is shown in Table 2. Final weight (1.19, 1.16, 1.18 and 1.15)), weight change (0.36, 0.34, 0.35 and 0.33), feed intake (0.89, 0.89, 0.89 and 0.89) and FCR (2.48, 2.72, 2.57 and 2.79) of pullets on 0 IU, 1000 IU, 2000 IU and 3000 IU, respectively were not significantly influenced (P>0.05) by the addition of cholecalciferol in the diets of the chickens. Pullets on zero supplementation of cholecalciferol had relative improvement in the measured parameters though were not significantly different (P<0.05) from other treatments.
|
|
|
4. Discussion
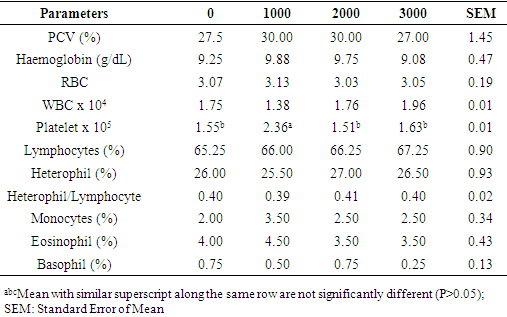
- Performance attributes monitored in this study were not affected by vitamin D3 supplementation. Feed was equally consumed by the pullets on the different levels of D3 indicating that dietary supplement of vitamin D3 did not influence their intake of feed. These were evident in the similarities observed in weight change, final weight and FCR. This observation implied that inclusion of supplemental D3 in the diets of pullets did not have any beneficial nor harmful effect on pullets performance. Similar report was documented for feed intake and final weight in growing pullets fed diets supplemented with ascorbic acid [23]. Earlier reports [32-34] on dietary supplementation of vitamin D3 in chickens equally revealed that performance traits were not affected when adequate levels of vitamin D3 were supplemented in diets of pullets. This could be an indication that the vitamin D3 requirement of the pullets must have been met and additional supplementation for the growing pullets did not exert any extra influence on their performance. However, other workers [35, 36] obtained significant increase in growth performance of chickens fed varying inclusion levels of vitamin D3 supplementation. Han et al. [37] also documented significant increases in weight gain, feed intake and improved FCR in broiler chicken with increasing dietary levels of vitamin D3 supplementation.Blood has been shown as an important index of physiological, pathological and nutritional status in animals [17, 20]. Aletor [19, 20] indicated that blood variables most constantly affected by dietary factors include RBC, PCV and plasma protein. The PCV range (27.00 to 30.00%) observed in this study, was higher than 24.67 and 27.00% reported by Talebi et al. [38] and similar to 30.07% reported by Oyewale [39] but was lower than 35.55% documented for caged chickens [40]. The PCV below 21-35% has been reported in poultry to be associated with anaemia, a condition attributed to intake of poor quality protein [41, 42]. Haemoglobin, the iron-containing oxygen-transport protein and the oxygen carrying capacity (RBC) of the blood remains an important component in the normal physiological functioning of cells. The Hb (g/dL) of 9.08 to 9.88 reported in this study was within the normal range of 7 to 13 [42, 43] for poultry. Index of haemoglobin has been used as an indication of nutritional anaemia in animals [44]. It may be used as a pointer to nutrient utilisation in diets since it has the physiological function of transporting oxygen to tissues in animals for oxidation of ingested food in order to release energy for body functions and transport carbon dioxide out of the body of animals [45]. Isaac et al. [46] opined that red blood cell is involved in the transport of oxygen and carbon dioxide in the body and a reduced red blood cell implies a reduction in the level of oxygen that would be carried to the lungs. The RBC which was in the range of 3.03 to 3.15 in this study were higher than the average reported in different strains of broiler chickens [38] and in broiler chickens fed diets containing varying levels of bitter Kola [47]. Vitamin D3 has been implicated in several immunological functions. Calcitriol has been shown to have immunoregulatory and immunomodulatory activity and directly affects all cells of the mononuclear lineage [48-51]. This metabolite inhibits growth of Mycobacteria tuberculosis in cultured human monocytes and macrophages [52, 53] and improves resistance to tuberculosis in a murine model [54]. Macrophages from vitamin D3-deficient mice functioned abnormally, and their function could be restored both in vitro and in vivo by treatment with 1, 25 (OH)2 D3 [55]. The major functions of the white blood cell and its differential are to fight infections, defend the body by phagocytes against invasion by foreign organism. Low differential counts can precipitate haematological abnormalities [56] which could undermine birds’ performance. As observed from this study, the WBC counts did not vary across treatments showing that dietary supplements did not influence the immune function of the pullets. The variation in components of leukocyte is indicative of external factors alien to the body of the animals. Observed similar values of heterophil, lymphocyte and heterophil/lymphocyte across treatments in this study showed that the test pullets possessed similar prowess to curtail invading aliens. Heterophil:lymphocyte is commonly used as an indicator of stress in chickens [57]. The presence of stress (nutritional, environmental, diseases etc) could cause variation in the population of heterophils and lymphocyte in the blood thus leading to an imbalanced ratio of heterophils:lymphocytes [57]. However, the similarity in the H:L of pullets across the treatment could imply that the supplementation did not impose any form of stress capable of disrupting the normalcy of the pullets’ immune system and as such the levels of supplementation in this trial were not in excess of dietary need so as to compromise their health. Although vitamin D3 has been implicated to have regulatory roles in immune cell functions [48], experiments have illustrated the successful treatments of autoimmune diseases with vitamin D3 in mice [58]. The deficiency of cholecalciferol has also been associated with a depression in cellular responses in young broiler chicks [59] while increment in supplemental vitamin D3 was associated with a 70% enhancement of lymphocyte proliferation [60]. Platelets also known as thrombocytes are cells involved in blood clotting. Reduction in number of platelets has been suggested to infer that the process of clot formation (blood clotting) would be prolonged thus resulting in excessive loss of blood during injury [61]. As observed in this study, supplementation of vitamin D3 at 1000 IU increased the potentials of the blood to clot in tested pullets, although supplementation beyond this level further lowered the clotting potential. Park et al. [62] had attributed increased platelet count to deficiency of vitamin D3 in human subjects which clearly indicated that dietary vitamin D3 has metabolic roles in platelet function in vivo. Cholecalciferol had been implicated in the mobilisation of Ca and P by authors [3, 12, 15] contrary to findings in this study where supplementation of vitamin D3 did not influence serum Ca and P. Han et al. [37] also had similar observation in broiler chickens. The authors noted that different levels of cholecalciferol did not influence serum Ca and P concentration. However, performance was reportedly improved by dietary supplementation of vitamin D3. The ALP activities were only increased at supplementation of 3000 IU D3 thus suggesting that the lower levels may not be sufficient to trigger further activities in the enzymes for mobilisation of phosphates, especially for bone growth in preparation of the pullets for the onset of lay. Although, cholecalciferol is closely involved in calcium metabolism, it is synthesised from 7-dehydrocholesterol which could possibly compensates for its deficiency by increasing cholesterol synthesis [63]. There was a gradual reduction in serum cholesterol with increasing levels of vitamin D3. Serum cholesterol was highest at 2000 IU vitamin D3 supplementation which however, was lowered with increasing supplemental levels. The HDL and LDL followed a similar trend as corresponding increases in the values of these parameters at 2000 IU D3 was related to high serum cholesterol at the same level. Effect of cholecalciferol on serum cholesterol composition has been inconsistent [63] though, authors reported a reduction in blood cholesterol in rowers administered vitamin D3 supplementation. Chow et al. [64] also reported cholesterol reduction in mouse given different levels of vitamin D3 supplementation. Supplementation of dietary cholecalciferol led to a progressive reduction in triglyceride levels in the serum. This corroborated the reported [65] reduction of blood lipids as < 5% changes in levels of lipids was adduced to vitamin D3 and calcium supplementation.
5. Conclusions
- It was concluded in this study that dietary supplement of vitamin D3 had no effects on performance attributes of growing pullets. Except for the serum lipids and platelets, the gross blood profile was unaffected. Supplemental cholecalciferol level of 2000 IU/kg may be beneficial to platelets and serum lipid compositions.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML