-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(3): 119-123
doi:10.5923/j.ijaf.20180803.01

Refractoriness of Porcine Corpora Lutea to Cloprostenol Sodium and Dinoprost Tromethamine Treatments at Day 7 of Oestrous Cycle
Oke-Egbodo B. E.1, Nwannenna A. I.2, Bawa E. K.1, Hassan R.1, Bello T. K.1, Bugau J.2, Oke P. O.3, Rekwot G. Z.1
1National Animal Production Research Institute, Ahmadu Bello University, Zaria, Nigeria
2Faculty of Veterinary Medicine, Ahmadu Bello University Zaria, Nigeria
3College of Veterinary Medicine, Federal University of Agriculture Makurdi, Nigeria
Correspondence to: Oke-Egbodo B. E., National Animal Production Research Institute, Ahmadu Bello University, Zaria, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This study was carried out to evaluate the refractoriness of porcine corpora lutea to exogenous prostaglandins (Cloprostenol sodium and Dinoprost tromethamine). Twenty (n = 20) apparently healthy sows were randomly assigned to 4 treatment groups. Group 1 (n=5) received two injections of Cloprostenol sodium (Synchromate®) (500µg) (days 0 and 13). Group 2 (n=5) received three injections of Synchromate® (Day 0, 7, 13). Group 3 (n=5) received two injections of Dinoprost tromethamine (Lutalyse®) (12.5mg) (days 0 and 13). Group 4 (n=5) received three injections of Lutalyse® (Day 0, 7, 13). Five (5) milliliters of blood was collected via the posterior vena cava before PGF2α injections on day 0, 7, and 13 and once weekly afterwards till pregnancy was established. Data on progesterone profile were expressed as mean ± SEM. One-way ANOVA and Tukey’s post hoc test were used to compare the mean values between the groups. Graphpad Prism® data package for windows (2009) was employed for all statistical analyses. A value of P < 0.05 was considered significant. There were no luteolysis seen following first and second injections of PGF2α in groups 2 and 4 and following first injections in groups 1 and 3. Complete luteolysis was seen with behavioural oestrus after the second injection of PGF2α at day 13 in groups 1 and 3 and after third injection of PGF2α in groups 2 and 4. It was therefore concluded that the corpora lutea was refractory to both Cloprostenol sodium and Dinoprost tromethamine at Day 7. Further work should be done to find out the reason for lack of response of porcine CL to PGF2α between days 7 and 12.
Keywords: Refractoriness, Cloprostenol Sodium, Dinoprost thromethamine, Porcine CL, Progesterone
Cite this paper: Oke-Egbodo B. E., Nwannenna A. I., Bawa E. K., Hassan R., Bello T. K., Bugau J., Oke P. O., Rekwot G. Z., Refractoriness of Porcine Corpora Lutea to Cloprostenol Sodium and Dinoprost Tromethamine Treatments at Day 7 of Oestrous Cycle, International Journal of Agriculture and Forestry, Vol. 8 No. 3, 2018, pp. 119-123. doi: 10.5923/j.ijaf.20180803.01.
Article Outline
1. Introduction
- Pig Production has been advocated as a short term measure towards alleviating the animal protein and calorie deficit, especially where there are no religious edicts preventing their production and consumption, (Ajala et al., 2007). Oestrus synchronization is a valuable management tool for increasing the pregnancy rate in pigs (Brüssow and Wahner, 2011). Several techniques have been developed to induce oestrus. Oestrus synchronization methods in the sow vary and are all based either on controlling events leading to follicular maturation and ovulation or altering luteal lifespan (Estill, 2000). Prostaglandin F2α (PGF2α) is not luteolytic in the sows until about day 12 of the oestrous cycle (De Rensis et al., 2012; Kouamo and Kamga-Waladjo, 2013; Tur, 2013). Synthetic progestins have been developed and used in sows for oestrus synchronization (Estienne et al., 2001; Van Leeuwen et al., 2011). The explanation for the relative insensitivity of porcine corpora lutea (CL) to the luteolytic effect of PGF2α prior to day 12 of the oestrous cycle is unknown (Estill et al., 1993). However, some studies demonstrated a relatively low numbers of specific high-affinity PGF2α-binding sites (receptors) on large luteal cells prior to day 12 (Gadsby et al., 1990; Diaz and Wiltbank, 2004). Others suggested that this lack of luteolytic sensitivity in porcine CL before Day 12 of the oestrous cycle may result from a deficiency in post-PGF2α receptor signaling that is activated within CL (Diaz et al., 2000). In addition, it was reported that the number of high affinity binding sites increased dramatically from day 13 coinciding with the apparent onset of enhanced sensitivity to the luteolytic effects of PGF2α between days 12 and 13 (Gadsby et al., 1990, Estill et al., 2000). The insensitivity of sow’s CL to the luteolytic effect of PGF2α before day 12 is considered to preclude the use of prostaglandin in oestrus synchronization programmes for swine (Przygrodzka et al., 2015). This study was therefore designed to evaluate the refractoriness of porcine corpora lutea to Cloprostenol sodium and Dinoprost tromethamine at day 7 of the sow’s oestrous cycle.
2. Materials and Methods
- Study LocationThe study was carried out at the Swine and Rabbit Research Programme of the National Animal Production Research Institute (NAPRI), Shika, Ahmadu Bello University, Zaria.Experimental Animals and Herd ManagementTwenty (n =20) apparently healthy cross bred sows belonging to the Swine and Rabbit Research Programme of the NAPRI Shika, Zaria were used for this study. The sows were between 2 - 3 years of age weighing between 120 and 150 kg and were identified with ear tag numbers. The cross bred sows were fed with diet containing 16% crude protein. The ration was formulated to meet the minimum nutrient requirements for breeding sows and boars as recommended by National Research Council (NRC) (1998). The ingredients for the diet were sourced in NAPRI feed store and the ration was mixed in the feed mill in NAPRI, Shika, Zaria. Water was given ad libitum.Experimental DesignA total of twenty (n = 20) cross bred sows were randomly divided into four groups and each of the group consists of 5 sows with different treatment protocol.Group 1 (n=5) – Double intramuscular injection of Cloprostenol sodium (Synchromate®). Each of the sows received a dose of 500 µg (2 ml) Cloprostenol sodium injection on days 0 (day of first injection) and 13 (day of second injection). The sows were then monitored for signs of oestrus. Those found in oestrus were bred using natural breeding.Group 2 (n=5) – Triple intramuscular injection of Cloprostenol sodium (Synchromate®). Each of the sows received a dose of 500 µg (2 ml) of Cloprostenol sodium on days 0 (day of first injection), 7(day of second injection) and 13 (day of third injection). The sows were also monitored for signs of oestrus. Those found exhibiting signs of oestrus were bred using natural breeding.Group 3 (n=5) – Double injection of Dinoprost tromethamine (Lutalyse®). Each of the sows received a dose of 12.5mg (2.5 ml) dinoprost tromethamine on days 0 (day of first injection) and 13 (day of second injection). The sows were then monitored for signs of oestrus. Those found in oestrus were bred using natural breeding.Group 4 (n=5) – Triple intramuscular injection of Dinoprost tromethamine (Lutalyse®). Each of the sows received a dose of 12.5mg (2.5 ml) of dinoprost tromethamine on days 0 (day of first injection), 7(day of second injection) and 13 (day of third injection). The sows were also monitored for signs of oestrus. Those found exhibiting signs of oestrus were bred using natural breeding.Oestrus Detection and MatingThe cross bred sows were observed visually for behavioural oestrus manifestation twice (0700-1000 and 1500-1800 h) daily from commencement of the study for 21 days. Sows were considered to be in oestrus when they stood to be mounted by females (homosexual mount) or male (heterosexual mount).Blood SamplingFive (5) milliliters of blood was collected via the posterior vena cava, using a 10-ml hypodermic syringe, fitted with 18 gauge needle, from the sows on days 0, 7, and 13 (just before prostaglandin injection), and once weekly afterwards until confirmation of pregnancy based on non-return rate to oestrus. Blood samples collected in vacutainers without anticoagulant were quickly transported to the laboratory. Serum samples were separated by centrifugation of the blood at 2500G for 15 minutes. The serum samples in vials were appropriately labeled and stored at -20°C until hormone analysis. Progesterone assaySerum P4 was determined by using Competitive ELISA kits (AccuBind® ELISA, Monobind Inc. 100 North Pointe Drive Lake Forest, CA 92630, USA), intended for a quantitative determination of P4 concerntration in serum or plasma using ELISA microplate reader (ELx800). The sensitivity of the assay was 0.105 ng/ml. Within assay precision, coefficients of variation for low, normal and high pooled controlled serum samples were 9.9%, 3.1% and 2.9% respectively. Data AnalysesData on progesterone profile during the oestrous cycle were expressed as mean ± SEM. One-way ANOVA and Tukey’s post hoc test were used to compare the mean values between the groups. Graphpad Prism® data package for windows (2009) was employed for all statistical analyses. A value of P < 0.05 was considered significant.
3. Results
- The serum P4 concentrations of the samples collected from the first day of PGF2α to 48 days or 26th day post- mating ranged from 0.00 to 11.87 ng/ml. For sows in group 1 (Figure 1), mean P4 concentration increased from 3.77 ± 1.53 ng/ml at first PGF2α (Synchromate®) to 6.73 ± 0.29 ng/ml on day 13th of the second dose of PGF2α (Synchromate®) injection the concentration. There was a decrease following the injection of the second dose of the Synchromate® to 0.63 ± 0.09 ng/ml at day 20 and an increase was seen at day 27 (6.60 ± 1.41 ng/ml) which continues to increase till 11.87 ± 0.20 ng/ml at day 48 of the treatment and 23rd day post mating. For sows in group 2 (n=5) (Figure 1), mean P4 concentration decreased from 7.13 ± 0.64 ng/ml at first PGF2α (Synchromate®) to 4.43± 1.72 ng/ml on day 7th of the second dose of PGF2α (Synchromate®) injection and increased to 6.57 ± 0.23 on day 13th before the third dose of PGF2α (Synchromate®) injection. There was a decrease following the injection of the third dose of the Synchromate® to 0.23 ± 0.09 ng/ml at day 20 and an increase was seen at day 27 (6.10 ± 1.01 ng/ml) which continues to increase till 11.93 ± 0.87 ng/ml at day 48 of the treatment and 24th day post mating. For sows in group 3 (Figure 1), mean P4 concentration increased from 4.8 ± 1.86 ng/ml at first PGF2α (Lutalyse®) to 7.00 ± 0.95 ng/ml on day 13th of the second dose of PGF2α (Lutalyse®) injection the concentration. There was a decrease following the injection of the second dose of the Lutalyse® to 9.87 ± 1.20 ng/ml at day 20 and 0.80 ± 0.36 ng/ml at day 27. An increase was seen at day 34 (5.50 ± 0.29 ng/ml) which continues to increase till 10.83 ± 0.75 ng/ml at day 48 of the treatment and 26th day post mating. For sows in group 4 (Figure 1), mean P4 concentration increased from 1.77 ± 1.23 ng/ml at first PGF2α (Lutalyse®) to 3.90 ± 0.38 ng/ml on day 7th of the second dose of PGF2α (Lutalyse®) injection and slightly decreased to 3.87 ± 1.86 ng/ml (though decreased but no luteolysis) on day 13th of the third dose of PGF2α (Lutalyse®) injection. There was a decrease following the injection of the third dose of the Lutalyse® to 0.63 ± 0.20 ng/ml at day 20 and an increase was seen at day 27 (4.30 ± 1.23 ng/ml) which continues to increase till 10.97 ± 1.01 ng/ml at day 48 of the treatment and 26th day post mating. Overall mean showed no statistical significant difference (P> 0.05) between the groups.
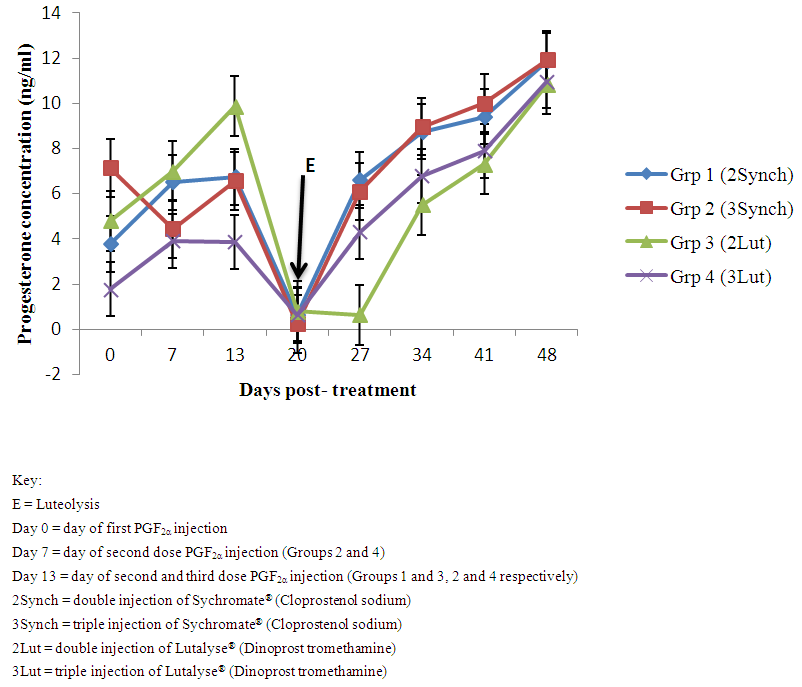 | Figure 1. Progesterone profile of cross bred sows treated with double and triple injections of PGF2α (Synchromate® and Lutalyse®) |
4. Discussion
- The results of this study have shown the luteolytic effect of Cloprostenol sodium (Synchromate®) and Dinoprost tromethamine (Lutalyse®) in porcine corpora lutea. In group 1, the basal concentration of P4 (3.77 ± 1.53 ng/ml) after the injection of PGF2α was an increased (6.50 ± 0.20 ng/ml) after one week of the injection this shows that most of the animals in group 1 were in their early stage of luteal phase making the CL unresponsive to the injected PGF2α as porcine CL is unresponsive to exogenous PGF2α befor day 12 which is in agreement with Guthrie and Polge, 1976, Estill et al., 1993, Zannoni et al., 2007, De Rensis et al., 2012 and Kouamo and Kamga, 2013. There was luteolysis following second dose of the PGF2α at day 13 (0.63 ± 0.09 ng/ml) with oestrus signs which is in corroboration with the luteolytic effect of PGF2α on porcine CL as reported by De Rensis et al., 2012. Pregnancy was established following increased P4 concentration (11.87 ± 0.20 ng/ml) which is in agreement with Boma and Bilkei (2008) who reported P4 concentration of pregnant sows to be greater than 5ng/ml.In group 2 with triple doses of synchromate®, the basal p4 level (7.13 ± 0.64 ng/ml) decreased to (4.43± 1.72 ng/ml) which indicate that the most of the animals were in their late luteal phase on the day of first PGF2α injection which was able to cause decrease in P4 concentration following the first dose of the injection but the second dose given on day 7 (4.43± 1.72 ng/ml) there was no reduction in the p4 rather an increased P4 was seen (6.57 ± 0.23 ng/ml) then a decrease in the p4 (0.23 ± 0.09 ng/ml) following the third dose of PGF2α due to the CL responsiveness to PGF2α from day 12 as reported by Estill et al., 1993 and Kouamo and Kamga, 2013 and oestrus was observed in the animals hence complete luteolysis occurred after the third injection of PGF2α. Following oestrus and natural breeding there was significant increase in the p4 concentration from mating till day 48 (11.93 ± 0.87 ng/ml) which indicates pregnancy as reported by (Boma and Bilkei, 2008).In group 3 the basal p4 concentration (4.8 ± 1.86 ng/ml) was elevated (7.00 ± 0.95ng/ml) after the first dose of PGF2α which indicates that luteolysis has not taken place and that the animals were in their early luteal phase and reports has shown non responsiveness of porcine CL to exogenous PGF2α as reported by De Rensis et al. 2012 and Kouamo and Kamga, 2013. Luteolysis (0.80 ± 0.36ng/ml) was reported following the second injection of PGF2α which is in agreement with that reported by De Rensis et al. 2012. Pregnancy was established (10.83 ± 0.75 ng/ml) which was also in agreement with that reported by Boma and Bilkei (2008).In group 4 (triple injection of Lutalyse®) the basal p4 concentration (1.77 ± 1.23 ng/ml) indicates that the animals were in their proestrus because no observable signs of oestrus was seen but following the first dose of PGF2α there was a slight though not significant decline in the p4 concentration (3.90 ± 0.38 ng/ml). Luteolysis with observable oestrus signs were reported in this study following the third injection of PGF2α (0.63 ± 0.20 ng/ml) which is in agreement with the works of De Rensis et al. 2012, Kouamo and Kamga, 2013 and Przygrodzka et al. 2015. Pregnancy was established (10.97 ± 1.01 ng/ml) which was also in agreement with that reported by Boma and Bilkei (2008).
5. Conclusions and Recommendations
- It is therefore concluded that porcine corpora lutea is refractory to exogenous PGF2α at day 7 of the oestrus cycle but was responsive following injection at day 13. Further work should be done to find out the reason for lack of response of porcine CL to PGF2α between days 7 and 12 and appropriate pregnancy diagnosis should be done to avoid early pregnancy loss as PGF2α is an arbotifacient.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML