-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(2): 98-103
doi:10.5923/j.ijaf.20180802.08

Dormancy in Seeds of Hybrid Cassava Varieties (TMS 98/0505 and TMS 95/0379) Prior to Hardening of Seed Coat
Esther E. Ugbede , Elsie I. Hamadina
Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Elsie I. Hamadina , Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Many tropical seeds commence dormancy at the formation of a hard seed coat. Although cassava seeds are viable before the formation of a hard seed coat, it is unclear whether they are dormant or not. Therefore, the objective of this study was to determine whether seeds of two cassava varieties (TMS 98/0505 and TMS 95/0379) collected before hardening of seed coat are dormant. Cassava fruits were randomly collected at 6 and 10 weeks after first anthesis. Seeds were tested for viability using tetrazolium chloride (TCL) and flotation tests. Filled seeds from floatation test were then treated in three concentrations (0, 10 and 30 µM) of fluridone (a dormancy releasing chemical, which acts by inhibiting the biosynthesis of the dormancy inducing hormone, abscisic acid) and observed for germination. Result from TCL test showed that 0.8 and 0.0 seeds per fruit were viable at 6 weeks after anthesis (WAA) in TMS 98/0505 and TMS 95/0379 respectively. However, at 10 WAA, 1.6 seeds per fruit were viable in TMS 98/0505. Thus, the probability of having viable seeds increased significantly (p<0.05) from 0.48 at 6 WAA to 0.92 at 10 WAA. Also, whether seeds were treated in fluridone or not (i.e. in the control), no germination was observed at 30 days after treatment. Thus, the seeds were more likely to be dormant (at p<0.001 with 95% confidence interval for proportion (0.9664, 1.000)) than non-dormant at both collection dates and in both varieties but the cause of dormancy may not be directly related to abscisic acid content. Thus, if the benefits of obtaining large number viable seeds at 10 WAA is to be derived more studies are required to determine the cause and cure of dormancy in immature cassava seeds.
Keywords: Seed formation, Tetrazolium test, Fluridon, Seed dormancy, Cassava variety
Cite this paper: Esther E. Ugbede , Elsie I. Hamadina , Dormancy in Seeds of Hybrid Cassava Varieties (TMS 98/0505 and TMS 95/0379) Prior to Hardening of Seed Coat, International Journal of Agriculture and Forestry, Vol. 8 No. 2, 2018, pp. 98-103. doi: 10.5923/j.ijaf.20180802.08.
Article Outline
1. Introduction
- Commercial production of cassava tuber is mainly through vegetative propagation of stem cuttings, which ensures the maintenance of genetic integrity, and reduces stand-to-stand variability. However, the productivity of stem cuttings is highly dependent on the quality of the cuttings (i.e., high yielding, resistant to common pest and diseases such as Cassava Mosaic Disease (CMD) and environmental conditions such as drought) [1]. Unfortunately, the process of developing quality stem cuttings of hybrid cassava is a long process. It begins with the screening of seedling populations with the aim of selecting superior plants and then cross breeding them with a desirable female. Cross breeding encourages the recombination of genes that increase the chance of producing genetically diverse and superior plants and seeds with desirable qualities. Thus, the rate at which new cassava hybrids are developed depends highly on the population of seedlings that can be raised from seed lots over a short period of time. Cassava seeds have the advantage of being easy to storage, maintain viability over prolonged storage, easy to transport and have a high multiplication rate [2]. The limiting factors of sexual propagation is lack of quick and uniform seed germination as well as the difficulties associated with cultivation of highly variable propagules [2, 3]. Long seed dormancy and poor seed germination are major problems slowing down the already painfully long process of breeding for cassava hybrids through hybridization [4, 3]. This implies that the pace of production of breeder’s stock for national seed service cassava multiplication program is slow [5, 6, 3]. If a large proportion of hybrid cassava seeds could be induced to germinate early enough, the pace at which new hybrids are produced will quicken and the problem pace of breeding could be averted [7]. Understanding of the physiology of cassava seed development and dormancy can help unravel the difficulties associated with germinating cassava true seeds. During cassava fruit development, the seeds become viable from one month after pollination while seed (with hardened seed coat) and fruit maturity occur one and two months later respectively [8]. Coincidentally, fruits are commonly collected at fruit maturity/ brown fruit stage, which coincide with the months of November-January depending on variety. Sometimes, they are collected just before the browning of the fruit to avoid open dispersal of the seeds. The commencement of dormancy is often linked to the formation of hard seed coat or to the start of fruit browning, and so, dormancy is said to last for 3-6 months or more depending on variety. Consequently, studies on cassava seed dormancy and germination have centered on periods after the formation of hard seed coat. Under this paradigm, dormancy is strongly attributed to the presence of hard seed coat (i.e. physical or morphological dormancy), the nature of the seed coat (i.e. impermeability to water or gas), factors of the environment such as temperature or a combination of these. Working under this paradigm, studies have shown that the exposure of seeds to moist or dry heat at temperature of 60°C promotes germination by synchronizing germination [4, 9]. Similar effect on germination is observed when seeds are stored at very low temperature of 4°C for up to one year enhances germination or when the hard seed coat is thinned down mechanically or chemically, although scarification is less effective than wet or dry heat treatments [4]. Therefore, because the difference in mean time from treatment to 60 or 80% germination is usually within the same range as that of the controls suggests that the set of seeds assessed were within the same physiological age; perhaps tending towards not being dormant, and that the treatments synchronize germination than that they break dormancy. Non-dormant, cassava seeds require about 16-21 days to germinate [8]. Furthermore, based on published data, these treatments do not increase germination by up to 40% in many cassava genotypes [9]. Thus, there is need to determine whether there are other causes of seed dormancy in mature cassava seeds. Also, since cassava seeds are viable well before seed maturity for harvest, it is important to determine whether such seeds are dormant. If they are not dormant, then they could be collected before the formation of a hard seed to avoid the need to follow the cumbersome and expensive process of scarifying. The collection of seeds before the formation of a hard seed (to avoid dormancy) is a common practice for recalcitrant seeds of tropical trees that exhibit dormancy [11]. Although it is commonly assumed that seeds are not dormant prior to hard seed coat formation, this has not been ascertained in cassava. This study seeks to answer the following questions: are viable cassava seeds collected prior to the formation of hard seed coat dormant? Since this study proposes that cassava seeds are dormant prior to the formation of a hard seed, this study also questions whether the inability of cassava seeds to germinate is related to the presence of growth inhibiting compounds such as abscisic acid (ABA)? Abscisic acid (ABA) is a well-known growth inhibitor known to play a prominent role in the control dormancy and germination [12, 13]. Generally, ABA accumulates in seeds during mid- and late-maturation stages [14], and the response of dormant plant parts to Fluridone (a well-known inhibitor of ABA biosynthesis through the inhibition of the conversion of phytoene to carotenoid, which is a precursor of ABA) has long been used to determine the role of ABA in dormancy. The objective of this study was to: 1) determine whether viable cassava seeds collected prior to the formation of hard seed coat are dormant; and 2) determine the effect of Fluridone and seed collection prior to the formation of hard seed coat on germinability of two cassava varieties (TMS 98/0505 and TMS 95/0379).
2. Materials and Method
2.1. Study Environment
- The field work was carried out on the Teaching and Research Farm, Faculty of Agriculture, University of Port Harcourt, Rivers State Nigeria, while treated seeds were cultured and observed in a propagator. The University of Port Harcourt is located between Latitude 4° 53’ 25’’ and 4° 54’ 35’’N and Longitude 6° 54’ 25’’ and 6° 55’ 55’’E. The rainfall distribution is nearly all year round though its intensity is seasonal and variable. The monthly mean maximum temperature ranges from 28°C to 33°C while the monthly minimum temperature ranges from 17°C to 24°C [15].The propagator was a 2.5 m x 1.5 m x 1.5 m (length x width x height) structure covered with white polythene film on all sides. Temperature and relative humidity inside and outside the propagator was monitored at 8 am and 4 pm daily during the experiment using Galaxy Sensor Model KT-908.
2.2. Cassava Varieties
- Two varieties of cassava were studied. These were Manihot esculenta var. TMS 98/0505 and Manihot esculenta var. TMS 95/0379. The stem cuttings of these varieties were collected from an onfarm site, Kpite in Tai Local Government Area of Rivers State, Nigeria. These varieties were chosen because the seeds express very long dormancy and flower profusely.
2.3. Production of Cassava Seeds and Fruit Collection
- Stem cuttings of the selected cassava hybrid varieties were grown on the farm for seed production. Fifty-one stem cuttings per variety were randomly grown in three blocks. Each stem cutting was 20 cm long, and they were planted on moulds at a planting distance of 90 cm × 100 cm for both varieties.Soil samples were collected randomly from each block at a depth of 0-15 cm. The samples collected from each block were mixed together to obtain one homogeneous sample per block. The following parameters were analysed: organic matter and carbon, soil pH, soil type (sand, silt and clay), nitrogen (N), phosphorous (P), potassium (K), calcium (Ca), magnesium (Mg). Fruit samples were collected twice: at least 1 month after first anthesis and 4 weeks later. At each sampling date, all the fruits/rep required for this study were collected and randomly assigned to the different assessment components of the study.
2.4. Fruit and Seed Assessment for Maturity
- At each sampling date, 15 fruits were randomly assigned to this aspect of the study. Fruit and seed assessment was done following the methods by [11]. The maturity attributes assessed were: fruit colour, hardiness of fruit, dehydration, and dehiscent/non- dehiscent, loosing. The attributes considered for seed assessment were: hardness of seed coat, hydration, seed coat colour, endosperm and embryo development.
2.5. Determination of Seed Viability
2.5.1. Flotation Test
- At each sampling dates, 15 fruits/var were sampled i.e., 5 fruits/var/rep, assigned to this aspect of the study. The seeds were extracted from the fruit and dropped in distilled water. This was useful in separating pseudo seeds (floating seeds) from filled seeds (submerged seeds).
2.5.2. Tetrazolium Chloride Test
- The filled seeds collected after the flotation test were pre- conditioned by soaking them in water for 24 hours. Seeds were dissected longitudinally. One half of each seeds was then soaked in 0.5% tetrazolium chloride solution for 6 hours. Seeds were observed under a microscope (Extech® Mini Microscope, Model MC 108). Stained or non- stained cotyledon and radicle were counted for the determination of percentage viable seeds. At the end of the study, un-germinated seeds were evaluated for viability.
2.6. Determination of Dormancy Status of Seeds Collected Prior to Hard Seed Coat Formation and Effects of Seed Collection Date and Fluridone on Germination
- At each sampling date, 36 fruits per variety (i.e., 12 fruits per variety per replication), were assigned to this aspect of the study. Seeds were extracted from the fruit and were sterilized using 1% Sodium Hypochlorite for 5 mins. Sterile distilled water was used to rinse the seeds two times after which the seeds were soaked in the different concentration of Fluridone (0, 10 µM and 30µM) for 6 hours. These concentrations were chosen because they were found to be useful in enhancing germination in other dormant tubers seeds of many plant species. Seeds from each fruit were placed in between moistened filter paper and allowed to germinate in petri dishes.
2.7. Data Collection
- On the field, date of shoot emergence was recorded and duration to 50% and 70% emergence were calculated. 5 plants per variety per block that emerged between 13th and 16th were randomly selected for assessment of plant height, number of stems and number of leaves per stem. Date of first sign of branching was recorded for both varieties. Date of anthesis was recorded to calculate duration (in days) from planting to anthesis. The colour of the ring around the base of the pedicel was used to select fruits for collection at the second sampling date.In the seed germination study, seeds were observed daily for emergence (germination) of radicle and plumule. Data obtained was used to evaluate the dormant status of the seeds and the effect of Fluridone on germination. The experiment was terminated 30 days after treatment.
2.8. Experimental Design and Data Analysis
- The experiment was arranged as a Completely Randomized Design with two factors (sampling data and fluridone) at two and three levels respectively. Thus, there were six treatment combinations. All Data analysis were run on Genstat Discovery Edition 4. To determine the state of dormancy of seeds collected at each sampling date per variety, a one sample binomial test was run. This was chosen because each seed was observed and recorded as Germinating or non-germinating/dormant. In this analysis, R1 (success data set) = set of non-germinating/dormant seeds). Success observations were assigned 1 while non-success observations (germinating observation) were assign 0. The non-germinating data set was set as ‘Success set’ because this study hypothesizes that seeds harvested prior to seed hardening stage are already dormant. Data analysis for the effect of sampling date and fluridone on germination was not performed because the all seeds did not germinate irrespective of sampling date and fluridone treatment.
3. Results and Discussion
3.1. Soil Condition Prior to Cultivation of Cuttings
- Soil analysis was carried prior to cultivation. The soil was a loamy sand soil, pH was 6.3, organic matter content was 2.51%, total nitrogen was 0.13%, available Phosphorus was 27.7 mg/kg, calcium, magnesium, Potassium and Sodium were 2.3 cmol/kg, 1.44 cmol/kg, 1.54 cmol/kg, 0.91 cmol/kg respectively.
3.2. Vegetative Growth Characteristics of the Cassava
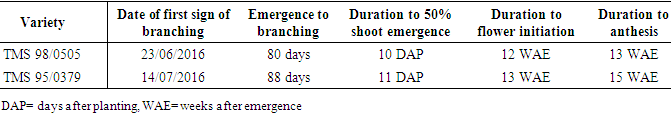
- The two varieties of cassava that were grown for seed production were different in terms of duration to 50% emergence, average duration to branching, date of first branching and date of first anthesis (Table 1). TMS 98/0505 was faster growing than TMS 95/0379; attaining 50% shoot emergence and first branching about one week earlier, and reaching anthesis two weeks earlier.
|
3.3. Experimental Conditions
- The average temperature inside and outside the propagator was 30.30°C and 32.17°C respectively in morning, and 29.70°C and 31.18°C respectively in the evening. The average relative humidity in the morning hour was 75% and, in the evening, it was 72%. The temperature and relative humidity were therefore slightly high but within acceptable range for germination.
3.4. Fruit and Seed Assessment for Maturity
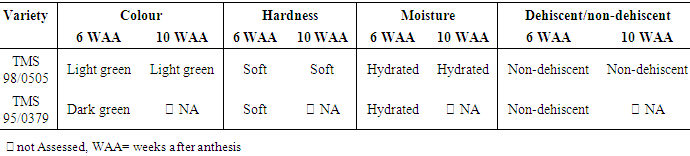
- At 6 WAA, the fruits of TMS 98/0505 were soft, hydrated, non-dehiscent, light green in colour (Table 2). Similarly, the fruits of TMS 95/0379 were soft, hydrated, non-dehiscent, dark green in colour. At 10 weeks after first anthesis, the fruit of TMS 98/0505 were still soft, hydrated, non-dehiscent, light green in colour and the colour of the ring around the base of the pedicel was yellow. TMS 95/0379 was not assessed at this date because the fruits were limited. The first sampling was done at 6 weeks after anthesis in TMS 98/0505 because TMS 98/0505 reached anthesis two weeks before TMS 95/0379 and so, two weeks after anthesis in TMS 98/0505 ensured that the fruits from TMS 95/0379 were collected at least one month after anthesis. The second sampling was done at 10 WAA in TMS 98/0505 to ensure that the fruits were collected at least two months after anthesis.
|
|
3.5. Viability of Seeds at 6 and 10 WAA
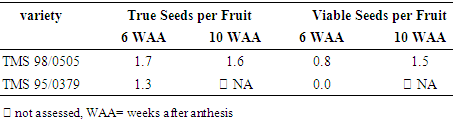
- Based on flotation test, the proportion of true seed/fruit was 1.7 and 1.6 at 6 WAA and 10 WAA respectively in TMS 98/0505 and 1.3 at 6 WAA in TMS 95/0379 (Table 4). However, tetrazolium chloride test showed that the seed viability per fruit in TMS 98/0505 was only 0.8 at 6 WAA and increased to 1.5 at 10 WAA while seed viability per fruit in TMS 95/0379 was 0% at 6 WAA. In percentages per variety, the tetrazolium chloride test showed that seed viability in TMS 98/0505 was 48% at 6 WAA and 92% at 10 WAA while seed viability in TMS 95/0379 was 0% at 6 WAA.
|
3.6. Dormancy Status of Seeds at 6 and 10 WAA
- Whether seeds were collected at 6 or 10 WAA, the seeds did not germinate 30 days after treatment in the control in TMS 98/0505 and TMS 95/0379. Also, since the seeds treated in 10 or 30 µM Fluridone did not also germinate; all observations were included in the test for the probability under a binomial distribution. Using the exact probability option, the test for null hypothesis that the proportion of success (non-sprouting/ dormant) is equal to 0.500 was significant at p<0.001 with 95% confidence interval for proportion (0.9664, 1.000). This result implies that the cassava seeds were dormant at both collection dates and in both varieties. Furthermore, it shows that dormancy in cassava seeds commences before the formation of hard seed coat. Although many hard seed coat forming seeds are not dormant prior to the formation of the hard seed coat [16], the induction of dormancy before the attainment of maturity is common in some plant species such as yam, allanblackia [23-25].
3.7. Effect of Fluridone on Germination
- Seeds of TMS 98/0505 and TMS 95/0379 did not germinate 30 days after treatment in the control and in 10 or 30µM Fluridone. Tetrazolium chloride test carried out at the end of the study (i.e. at 31 days after treatment) showed that the non-germinating seeds were not viable. This result is in contrast with the work of [24, 26] and many others, who showed that fluridone, an inhibitor of ABA biosynthesis, effectively broke seed dormancy in Allanblackia Spp. and C. tubulosa respectively. The inability of the seeds to germinate suggests that dormancy in cassava may not be due to the presence of ABA as speculated in this study.
4. Conclusions
- This study has shown that viable seeds can be obtained before the formation of hard seed coat as seen in TMS 98/0505 and that seeds can be collected as early as 10 WAA. Also, cassava seeds are dormant before seed coat hardens. However, the dormancy may not be related to the presence of the growth inhibitor, abscisic acid. In the light of these observations, further work is required to evaluate the role of other factors in the maintenance of dormancy in viable cassava seeds prior to the formation of a hard seed coat. The role of other physiological factors such as ‘the nature of the embryo not to germinate’ needs to be evaluated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML