Godson D. Nnedue, Elsie I. Hamadina
Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Seeds from mature cassava (Manihot esculenta Crantz) fruits exhibit low seed viability (one viable seed/fruit), possess hard seed coat and germinate poorly. Since the formation of hard seed coat is associated with the desiccation/last stage of seed development, we wondered whether cassava seed viability was related to seed moisture content. Therefore, the objectives of this study were: 1) to quantify the viability of seeds of two cassava varieties (TMS 98/0505 and TMS 95/0379) collected prior to the formation of hard coat, 2) to determine the relationship between seed viability and seed moisture content, and 3) to determine the suitability of top soil, moist filter paper, fine sand as growth medium for germinating young cassava seeds. Stem cuttings of the two varieties were cultivated on the field for seed production. Fruits were sampled two times at 6 and 10 weeks after first anthesis. Seeds extracted from the fruits had either thin, colorless seed coats or moderately thickened, light brownish seed coat. The seeds were subjected to flotation and tetrazolium chloride (TCL) tests for viability, evaluated for moisture content and subjected for germination in three growth media (top soil, fine sand and moist filter paper). Seed viability increased significantly (p<0.01) from approx. one seed/fruit (0.50) at 6 WAA to approx. all seeds per fruit (0.92) at 10 WAA in TMS 98/0505. In TMS 95/0379, seed viability was 0 at 6 WAA. Flotation test was found to overestimate seed viability at 6 WAA in both varieties. In TMS 98/0505, moisture content per seed significantly (p<0.01) declined from 0.80 g/seed to 0.50 g/seed as collection date was delayed from 6 WAA to 10 WAA. Moisture content was generally higher in TMS 95/0379 than TMS 98/0505. There was no interaction effect. The relationship between seed moisture content and seed viability was significant (p<0.001), and strongly linear (R²=0.89) and negative. There was no record of germination in the three-growth media which suggest that greater than one viable seed/fruit can be obtained before the formation of hard seed coat in TMS 98/0505 but methods that can germinate them needs to be developed.
Keywords:
Cassava hybrids, Seed dormancy, Seed viability, Seed desiccation, Germinability, Anthesis
Cite this paper: Godson D. Nnedue, Elsie I. Hamadina, Role of In Situ Seed Desiccation in the Control of Seed Viability of Cassava (Manihot esculenta crantz) Hybrids TMS 95/0379 and TMS 98/0505, International Journal of Agriculture and Forestry, Vol. 8 No. 2, 2018, pp. 92-97. doi: 10.5923/j.ijaf.20180802.07.
1. Introduction
Cassava (Manihot esculenta Crantz), is a member of the family Euphorbiaceae. It is a tuberous, woody perennial shrub that is propagated by vegetative means in tropical and sub-tropical regions of the world. It is very hardy; tolerates drought better than most other crops, and can grow well in very poor, acidic soils. In the tropical and sub-tropical regions, it is the third most important source of calories and the second most important food crop after maize in terms of global annual production [1, 2]. The tuberous root, which is the organ of economic importance, can be harvested even up to three years after attaining maturity [3]. The nutritional value of the storage root is mainly caloric even though it contains a lot of water, fiber, ash and protein [4]. In addition, the leaves contain about 30% proteins by dry weight and it is eaten in some parts of Africa as vegetable [5]. The tuber is a major source of income for most rural dwellers where it is processed into garri, starch, and animal feed or cassava chips. It is also sold to industries for the manufacture of alcohol, cosmetics, medicines and textile [6]. Consequently, the demand for quality cassava root is high.Conventional breeding for improved cassava root is constrained by some intrinsic factors namely high levels of genetic heterozygosity, variable flowering patterns, low seed viability and seed dormancy [7, 8]. These problems cause bottlenecks in the development of large populations of new cassava hybrids [9, 10]. Measures that curtail the negative impacts of these factors are therefore highly sought, and urgently so, following the current intensification of the cassava value chain around the world.Understanding the cause(s) of low seed viability (only one in three seeds per mature fruit) and surmounting them will highly increase the population viable seeds (Breeder’s stock) and seedlings. This research is therefore focused aspects of cassava seed physiology that facilitate the identification of possible causes of low seed viability in mature cassava. Cassava seeds are known to be viable about two months after pollination and so, the seeds are viable about two months before fruit maturity. Yet, mature seeds collected from mature fruits show such low viability. Furthermore, germination studies have shown that only 5-10% of seeds grown eventually germinate. While non-germinability of viable seeds under favorable environmental conditions is attributed to dormancy, the causes of low viability in cassava are less clear.The role of in-situ seed desiccation in the determination of the proportion viable cassava seeds is not known. In many recalcitrant seeds, an important cause of low seed viability is in-situ seed desiccation [11]. Loss of seed viability tends to occur as moisture content reduces below 12-30%. Recalcitrant seeds, which are desiccation-sensitive, tend to lose viability when they dry up during seed development to moisture content below a relatively high critical value. According to [12], cassava seeds are recalcitrant, but this was based on an analysis of ex situ desiccation. In their study, seed moisture content declined from 5.9 to 1.9% after 6 months in storage under laboratory temperature, and germination was observed to reduce from 80% to 20%. Although further research and examination of cassava seed germination after storage at 6-7 percent moisture content (i.e. desiccation was done ex situ) casted doubt on the recalcitrant status of cassava seeds [13], more research is required to verify this as well as evaluate the role of in situ desiccation on cassava seed viability.Therefore, this study posed the following questions: 1). are hybrid cassava seeds collected before seed coats hardening viable? 2). do cassava seeds loss moisture as the seeds progress towards maturity? Is the proportion of viable seeds per fruit affected by date of seed collection (collection before or after fruit maturity)? Is there a relationship between the loss of moisture and loss of viability? Can seeds obtained prior to fruit maturity germinate? The objectives of the study were thus: 1). To determine the relationship between seed moisture content of two cassava varieties (TMS 98/0505 and TMS 95/0379) and seed viability using the tetrazolium chloride test, 2) To determine the effect of date of seed collection on the proportion of viable seeds produced per fruit of two cassava varieties (TMS 98/0505 and TMS 95/0379) and 3) To assess the suitability of top soil, moist filter paper, fine sand as growth medium for germinating young seeds of two cassava varieties, (TMS 98/0505 and TMS 95/0379) collected before seed coats harden/fruit maturity.
2. Materials and Method
2.1. The Study Environment
Stem cuttings of selected hybrid cassava varieties were grown on the Faculty of Agriculture Teaching and Research Farm, University of Port Harcourt for seed production. Collected seeds were observed for germination in a propagator made of polyethylene film. The propagator was a 2m x 1m x 1 m (length x width x height).
2.2. Cassava Varieties
Two hybrid varieties of the Tropical Manihot Series TMS 98/0505 and TMS 95/0379 were used in this study. These varieties were chosen because the seeds exhibit long dormancy with poor germination particularly in TMS 98/0505 where percentage germination is between 5% and 12%. They also exhibit earliness in flowering and fruiting, they are high yielding and resistant to cassava mosaic disease as well as many other major pests of cassava [8, 14]. The stem cuttings of these varieties were collected from IITA on-farm trial site in Kpite, Tai Local Government Area of Rivers State Nigeria.
2.3. Cultivation of Cuttings
Twenty centimetres long stem cuttings of the two varieties were planted 5th of May 2016 at an inter-row and intra-row spacing of 1 m X 0.9 m. A total number of 51 plants per variety per block were planted.
2.4. Soil Analysis
Soil samples (collected at 0-15cm depth) were homogenized and analysed for organic matter content, total carbon, pH, total nitrogen (N), phosphorus (P), potassium (k), calcium (Ca) and magnesium (Mg) and soil physical characteristics.
2.5. Vegetative Growth Data Collection
Data collected were shoot emergence dates, date of first sign of flowering (flower bud initiation) at the main stem, first & second branching, branch height at first branching of the stem, as well as the date of anthesis of floret. Shoot emergence data was collected daily beginning from one week after planting and continued progressively until 90% germination was attained. The following were estimated: duration from 50% emergence to first branching, 50% emergence to flower initiation, average dates of pollination. Pollination was estimated to occur about 15 days after anthesis. This was also used to calculate the duration to first forking/branching. Five (5) plants were sampled per variety per rep at five weeks after planting for the collection of data on plant height, number of stems, and number of leaves per stem. Shoot emergence data were collected daily, plant heights weekly up to two (2) months, number of stem collected weekly until 90% emergence then monthly thereafter. Dates of first branching/forking and dates of first anthesis were based on weekly observations.
2.6. Sampling Date on Seed Viability
2.6.1. Floatation Test
At both sampling dates (6 & 10 WAA), 15 fruits/variety were sampled i.e., 5 fruits/variety/rep. Seeds were extracted from fruits and dropped in distilled water. This was useful in separating pseudo seeds (floating seeds) from true seeds (submerged seeds).
2.6.2. Tetrazolium Test
Fifteen fruits/variety (5 fruits /variety/rep) obtained from the field were sampled and preconditioned by soaking them in water for 24 hours at a temperature of 30-35°C. The seeds were dissected longitudinally through the embryo with a razor blade. Sectioned seeds were placed in 0.5% tetrazolium chloride solution for 6 hours and observation was made under a microscope to count stained cotyledon and radicle or plumule for the determination of percentage viable seeds.
2.7. Determination of Seed Moisture Content
Sampling was also done twice at 6 weeks and 10 weeks after anthesis respectively. Fifteen fruits/variety were sampled; 5 fruits/variety/rep. Fresh weights of each seeds were taken and dried in forced air oven at 100°C for 4 hours until constant weight. The dry weights of the seeds were recorded for the determination of seed moisture content. At 10 weeks after anthesis sampling was done from only one variety (TMS 98/0505) because fruits from the other variety (TMS 95/0379) were limited.
2.8. Germination Medium
Sampling was done at 6 weeks and 10 weeks after anthesis. At 6WAA, 45 fruits/variety (15 fruits/variety/rep) were sampled. Three (3) growth media (top soil, moist filter paper, fine sand) and two varieties (TMS 98/0505 and TMS 95/0379) were used. Seeds were extracted from the fruits (5 fruits/variety/medium/rep), soaked in distilled water for 1 hour and then 15 Seeds were sown/variety/medium. At the second sampling date, 45 fruits (15 fruits/block) from only one variety TMS 98/0505 were sampled. This was because fruits from the other variety TMS 95/0379 were limited. However, the seeds did not germinate all through the duration of the study.Experimental Design and Data AnalysisThe experiment on the effect of sampling date on viability of seeds of two cassava varieties was arranged as a Completely Randomized Design (CRD) with two factors at two levels each. The percentage viability was calculated using the formula: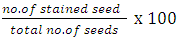 | (1) |
Proportion of true seeds/ fruit was calculated thus: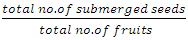 | (2) |
Proportion of viable seeds/ fruit was calculated thus: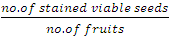 | (3) |
Data should have been analysed using a two-way ANOVA run on Genstat Discovery Edition 4 however, a two sample (seed viability at sample date 1 and sample date 2). Binomial test was carried out on Genstat Discovery Edition 4 because fruits of TMS 95/0351 were not sampled at 10 WAA. Stained and unstained seeds were given numbers 1 (stained) or 0 (unstained) prior to data analysis.The experiment on the effect of sampling date on moisture content of seeds of two cassava varieties was arranged as a Completely Randomized Design (CRD) with two factors at two levels each. Moisture content/seed was calculated as: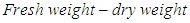 Percentage Moisture content was calculated as
Percentage Moisture content was calculated as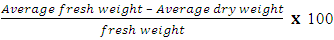 | (4) |
Data was analysed using a two-way ANOVA run on Genstat Discovery Edition 4. The design was a 2 X 2 factorial (4 treatment combinations) replicated 3 times. This software analysed the data as an unbalanced regressed model and predicted the mean value for the missing data points (i.e., 10 WAA, TMS 95/0357).The experiment on the effect of growth medium on germination of viable seed was arranged as a Completely Randomized Design with two factors; at two and three levels respectively. Thus, it was a 2×3-factorial with three replicates. Thus, there were 6 treatment combinations. The derived data were then analysed using a two-way ANOVA run on Genstat Discovery Edition 4. Means were separated using standard error of difference (SED) at p≤0.05.
3. Results and Discussion
3.1. Soil Characteristics
Results show that the soil type in the study location was as a loamy sand with a pH of 6.3, organic matter content of 2.51%, total organic Carbon was 1.46%, total organic Nitrogen was 0.173%, available Phosphorus was 27.7 mg/kg, Calcium, Magnesium, Potassium and Sodium were 2.3 cmol/kg, 1.44 cmol/kg, 1.54 cmol/kg, 0.91 cmol/kg respectively.
3.2. Vegetative Characteristics of the Cassava Varieties
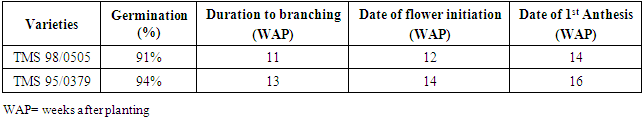
Cuttings of two cassava varieties (TMS 98/0505 and TMS 95/0379) had over 90% emergence; (Table 1). The average duration from planting to branching/forking varied between the two varieties; 11 WAP in TMS 98/0505 and 14 WAP in TMS 95/0379. The period of branching indicates a switch from vegetative growth to reproductive growth and hence, flower bud initiation. The average duration to flower initiation in TMS 98/0505 was 12 WAP and two weeks later in TMS 95/0379. The date of first anthesis was 14 WAP and 16 WAP in TMS 98/0505 and TMS 95/0379 respectively.Table 1. Vegetative characteristics of two Cassava varieties
 |
| |
|
3.3. Seed Growing Condition
Collected seed were germinated in a propagator. Morning temperature and relative humidity were 31-32°C and 74% respectively. The temperature outside the propagator was only slightly higher (32.3°C) than that inside (31.2°C). In the evening it was 29.5°C inside and 30.9°C outside with humidity of 73%. These conditions were warm and moist for seed germination.
3.4. Seed Moisture Content
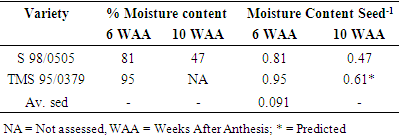
Due to the two-week difference in duration to first anthesis, the first sampling was done at 6 weeks after first anthesis (WAA) in TMS 98/0505, while the second sampling was done at 10 WAA in TMS 98/0505. This way, seeds in both varieties would be at least 4 weeks old. The moisture content of seeds of both varieties (TMS 98/0505 and TMS 95/0379) was high (above 80%) at the first sampling date (6 WAA) (Table 2). However, seed moisture content was higher in TMS 95/0379 than TMS 98/0505. At the second sampling (10 WAA), the moisture content reduced to 47% for TMS 98/0505, while TMS 95/0397 couldn’t be accessed due to insufficient fruits. Table 2. Moisture content of cassava varieties at 6 and 10 WAA
 |
| |
|
The test of significance for the difference in moisture content across variety and sampling date shows that average moisture content per seed significantly (p<0.001) declined by 0.33g by 10 WAA in TMS 98/0505 (Table 2). In TMS 98/0505, the moisture content per seed was predicted to significantly (p<0.01) declined by 0.34g by 10 WAA. Also, TMS 98/0505 had significantly (p<0.01) more moisture per seed than TMS 95/0379. There was no significant interaction effect.The significant findings of this study was the observation that up to 90% viable seeds could be obtained from the extremely poorly germinating variety of cassava (TMS 98/0505) by collecting the seeds at 10 WAA (i.e. just before the attainment of the hard seed coat). Further, this occurrence was found to be strongly related to seed moisture content. This study also shows that flotation test may not adequately represent true viability particularly when seed are collected prior to seed/fruit maturity.
3.5. Seed Viability
Tetrazolium chloride test and flotation test were used to determine viability of seeds of the two cassava varieties (TMS 98/0505 and TMS 95/0379) at two sampling dates (6 WAA and 10 WAA). Based on flotation test at 6 weeks after anthesis, the proportion of true seeds per fruit was 1.7 for TMS 98/0505 and 1.3 for TMS 95/0379 (Table 3). This indicates that the proportion of true seeds found in every fruit sampled for TMS 98/0505 was approximately two seeds per fruit and one for TMS 95/0379. At the second sampling date (10 WAA) the proportion of true seeds for TMS 98/0505 was 1.6 while that for TMS 95/0379 was not accessed because fruits were limited. In TMS 98/0505 therefore number of true seeds declined slight with later collected date.Table 3. Viability of seeds of cassava varieties using floatation (true seeds) and tetrazolium test (viable seeds)
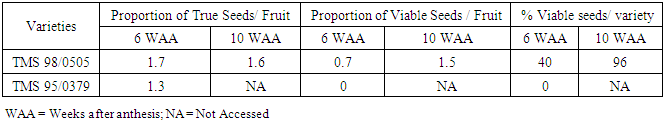 |
| |
|
On the other hand, the proportion of viable seeds per fruit based on the tetrazolium chloride test showed that seed viability fruit was 0.7 in TMS 98/0505 and 0 in TMS 95/0379. This implies that at 6 WAA the proportion of viable seeds was approximately one per seed per fruit for TMS 98/0505 and nil for TMS 95/0379. The proportion of viable seeds increased at the second sampling date (10 WAA) to 1.5, which is approximately 2 seeds/fruit for TMS 98/0505. The percentage seed viability per variety (TMS 98/0505 and TMS 95/0379) at 6 WAA was 40% and 0% respectively while at 10 WAA the percentage viability of TMS 98/0505 increased by 56%. Seeds of TMS 95/0379 were not assessed at 10 WAA (Table 3). To test whether the observed effect of sampling date on seed viability was significant, a two sample (seed viability at sample 6 WAA and 10 WAA) binomial test was carried out on Genstat Discovery Edition 4. This statistical analysis became necessary since seed viability was not assessed in TMS 95/0379 at 10 WAA. Result showed that, seed viability in TMS 98/0505 increased significantly (p=0.01) from 0.48 to 0.92 with sampling at 6 WAA and 10 WAA respectively (Table 4).Table 4. Effect of sampling date on seed viability of TMS 98/0505
 |
| |
|
Date of seed collection is shown strongly affect the proportion of viable seeds per fruit in the two cassava varieties (TMS 98/0505 and TMS 95/0379). Based on the tetrazolium test for viability, it was observed that the proportion of viable seeds per fruit increased with the date of seed collection. At an earlier sampling date i.e. at 6 weeks after anthesis, the proportion of viable seeds per fruit was approximately 1 in 3 seeds per fruit for TMS 98/0505 and none for TMS 95/0379. This increased subsequently with the second sampling date at 10 weeks after anthesis.
3.6. Seed Viability vs. Seed Moisture Content
Figure 1 shows the relationship between the viability and moisture content of the two varieties. The relationship between seed moisture content and seed viability was significantly (p=0.001) linear and negative with an R value of 0.89. As moisture content decreased at the second sampling date (10 WAA), there was a corresponding increase in seed viability per fruit and vice versa.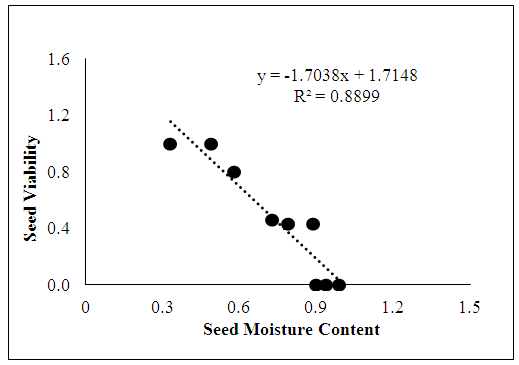 | Figure 1. Relationship between moisture content and seed viability |
The proportion of viable seeds per fruit increased to approximately all 3 seeds per fruit in TMS 98/0505. This implies that the proportion of viable seeds per fruit in TMS 98/0505 increased with delay in date of seed collection. This result agrees with the work of [15] who showed that viability was lowest in kenaf at 15 DAF (days after flowering), but it increased with progressing seed development. This is also in line with the suggestions that seed viability/quality is at the maximum at the end of seed filling phase [16-18].Although cassava seeds are said to be viable at 4 WAA as the embryos are mostly well formed at this age, it is clear from this work that this may not be the case for all varieties (i.e. in TMS 98/0379 which were collected at precisely 4 WAA). Even in TMS 98/0505 viability was low (only about 1 in 3 seeds/fruit) at 6 WAA. Thus, 10 weeks after anthesis appears therefore to represent an important date for seed collection in cassava. This date is proposed because cassava seeds collected before the 10th week after anthesis or much later at fruit maturity (when seed coats are brown and hard), are generally low in germinability.Moisture content was found to be very high in both varieties of cassava (80% and 95% for TMS 98/0505 and TMS 95/0379 respectively) when seeds are collected too early i.e. at 6 weeks after anthesis. However, at a later sampling date i.e. at 10 weeks after first anthesis, the percentage moisture content reduced to 49% in TMS 98/0505, the relationship between seed moisture content and seed viability clearly shows that in situ desiccation occurs during cassava seed development. We propose therefore that in situ desiccation up to a critical (perhaps between 35-45% moisture content) is necessary to maintain cassava seed viability at 80-100% while further in situ desiccation may cause reduction in seed viability. This critical moisture content range is suggested because cassava seeds are known to have even lower moisture content at fruit maturity, and seeds collected at fruit maturity are usually brownish, possess very hard seed coat and exhibit very low germination [8]. This suggestion is further supported by the fact recalcitrant seeds (of which cassava proposed to be one), are known to loss viability with in-situ seed desiccation below a critical value [11].
4. Conclusions
Cassava breeding is constrained by certain factors, including seed viability and dormancy, which hampers the development of large populations of new cassava hybrids. Hence, measures to eliminate the constraints are needed to support the intensification of cassava production globally. Discerning and surmounting the cause(s) of low seed viability will enhance breeding by ensuring availability of viable seeds. The findings of this study show that cassava seeds collected prior to hardening of the seed coat are viable and the seeds loose moisture as they progress towards maturity. The relationship between seed moisture content and viability is strong, linear and negative. Also, in situ desiccation up to a critical range (perhaps between 35-45% moisture content) is necessary to maintain cassava seed viability at 80-100%, while further in situ desiccation may cause reduction in seed viability. Collecting cassava seeds at 10 WAA, the proportion of viable seeds/fruit is greater than what is generally reported, (i.e., 1 seed/per when collected after full fruit maturity). Thus, 10 weeks after anthesis appears therefore to represent an important date for seed collection in cassava. Further work is required to establish the best medium for germinating the viable seeds obtained at 10 WAA. The finding from this work will help increase the population of the breeding stock of cassava breeders.
References
| [1] | Ceballos, H., C.A. Iglesias, J.C. Pérez, and A.G.O. Dixon. 2004. Cassava breeding: opportunities and challenges. Plant Molecular Biology 56: 503-515. |
| [2] | Food and Agricultural organization (FAO). (2010). Statistical database of the food and agricultural organization of the United Nations available at http://faostat. |
| [3] | Lebot, V. (2009), “Tropical Root and tuber crops: cassava, sweet potato, yams, and Aroids”, crop production science in Horticulture series vol. 17, pp. 1-433. |
| [4] | Marcelis, L.F.M, and Heuvelink, E. (2002). The latest developments in the lightning technologies in dutch horticulture. ActaHort 580, 35-42. |
| [5] | Burns H.K. Redolf K.L and Genos K.M (2010). Cropping systems in cassava productivity. pages 50-55. |
| [6] | IITA, (1990). Cassava in Tropical Africa. A reference manual. Balding and Mansell International, Wesbech, UK. p.176. |
| [7] | Jennings, D.L., Iglesias, C.A. (2002). Breeding for crop improvement. In: R.J. Hillocks. |
| [8] | Njoku, D.N, Ikeogu, U.N, Ewa, F Egesi. C, (2015). Crossability and germinablity potentials of some cassva (Manihot esculenta Crantz) progenitors for selection. National Root Crop and Research institute (NRCRI) Umudike, P.M.B. 7006, Umuahia, Abia state, Nigeria. Vol. 7(3), pp. |
| [9] | Jennings, D.L. (1963). Variation in pollen and ovule fertility in varieties of cassava, and the effect of interspecific crossing on fertility. Euphytica12: pages 69-76. |
| [10] | Byrne, D. (1984). Breeding cassava. Plant Breeding Reviews Journal, vol; 73-133 pages 30-34. |
| [11] | Roberts EH (1973). Predicting the storage life of seeds. Seed Sci Tech 1:499–514 United Kingdom, pp. 67-89 Wallingford, United Kingdom, pp. 1-16. |
| [12] | Mumford, P.M. and Grout, B.W.W. (1978). Germination and liquid nitrogen storage of cassava seed. Ann. Botany42, 255-257. |
| [13] | Ellis RH, Roberts EH (1979). Towards a rational basisfor testing seed quality. In: Seed Production (Heb-blethwaite PD, ed) Butterworths, London, 605-635. |
| [14] | Dixon, A.G.O; Okechukwu, R.U; Akoroda, M. O; llona, P; Ogbe, F; Egesi, C.N; Kulakow, P; Makuk G.S.S; Maziya-Dixon; lluebbey, P; Ypmeni, M.O; Geteloma, C; Jamews, B; Eke-okoro, O.N; Sanni, L; Tawuruhunga, N.P; Tarawali, G; Mahungu, N; Lemchi, J; Ezedinma, C.I; Okoro; E, Kanju, E; Adenji, A.A and Nwosu. K, (2010). Improved cassava variety handbook. IITA, Ibadan, Nigeria. |
| [15] | Olasoji J. O., Aluko A. O., Adeniyan O. N., Olanipekun S. O., Olosunde A. A. and Okoh J. O. (2012). Effect of time of harvest on physiological maturity and kenaf (Hibiscus canabinus) seed quality Afri. J. of Plant Sci., 6(10): 282-289. |
| [16] | Harrington, J.F. (1972). Seed storage and longevity, p. 145-245. In: T.T. Kozlowski (ed.). Seed biology, vol. III. Academic. New York. |
| [17] | TeKrony, D.M. and J.L. Hunter. (1995). Effect of seed maturation and genotype on seed vigor in maize. Crop Science 35: 862-868. |
| [18] | TeKrony, D.M. and D.B. Egli. (1996). Accumulation of seed vigor during development and maturation. p. 369-385. In: R.H. Ellis, M. Black, A.J. Murdock and T.H. Hong (eds.). Basic and Appllied Aspects of Seed Biology. Kluwer Academic Pub. London, UK. |


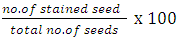
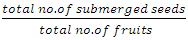
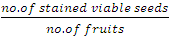
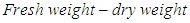 Percentage Moisture content was calculated as
Percentage Moisture content was calculated as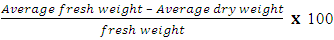

 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML


