Havva Dinler, Merve Günay
Department of Plant Protection, Faculty of Agriculture and Natural Sciences, Usak, Turkey
Correspondence to: Havva Dinler, Department of Plant Protection, Faculty of Agriculture and Natural Sciences, Usak, Turkey.
| Email: |  |
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
Healthy seeds are highly important in greenhouse vegetable growing in order to enhance the yield per unit area and get quality crops. Therefore, it is necessary to detect various disease factors transported with seeds and management with these factors. Fungi are also one of the significant factor groups which can be transported with seeds and cause economic crop losses in greenhouse vegetable growing fields. This study was conducted under in-vitro conditions in August and October in 2016 in order to detect fungal pathogens in some vegetable seeds (okra, eggplant, tomato, pepper, cucumber, bean, cowpea, etc.) in greenhouse vegetable growing fields in Usak province. For this purpose, naturally infected vegetable seeds were obtained from the fruits of vegetables of the previous year collected by farmers from the cultivation fields. Techniques of DFB (deep-freezing blotter) and AP (agar plate) methods suggested by International Seed Test Association (ISTA, 1996; Mathur and Kongsdal, 2003; Al-Askar et.al., 2014) were used in identifying the seed borne fungal flora. Aspergillus niger, Penicillium spp., Rhizoctonia sp., Phytophthora spp., Fusarium sp. Alternaria sp., Curvularia sp., Cylindrocarpon sp., Cladosporium sp., Epicoccum sp. Botrytis cinerea, Mucor sp., Macrophomina phaseolina and Rhizopus stolonifer agents were identified in vegetable seeds in general.
Keywords:
Seed borne pathogens, Vegetable, Seed, Green house
Cite this paper: Havva Dinler, Merve Günay, Determination of Fungal Agents in Some Vegetables Seeds in Greenhouse Production Areas in Uşak Province, International Journal of Agriculture and Forestry, Vol. 8 No. 2, 2018, pp. 83-91. doi: 10.5923/j.ijaf.20180802.06.
1. Introduction
Food requirement has also been increasing along with the increasing world population and it is necessary to enchance agricultural yield and have more amount of crops from unit areas in order to meet this food requirement. Since our country has appropriate ecological conditions for vegetable growing, this enables many species of horticulture to grow. It is also possible to grow various species of vegetable through greenhouse growing. Turkey is among the self-sufficient countries in terms of vegetable production. Our country is the first in Europe and the fourth country in the world with approximately 27.5 million tons of production (Abak, 2012). Greenhouse vegetables in our country are intensively grown primarily in the Mediterranean Region and then in Aegean and Marmara Regions. In Usak province greenhouse vegetables are grown in 687 areas and approximately 7.807 tons of crop is obtained. Most of the greenhouses are of high tunnel types. Greenhouse vegetables are intensively grown in Banaz in Usak province. Enterprises are small scale enterprises and 5.311 tons of the production is tomatoes, 1.204 tons is cucumbers and the rest is mixed types of vegetables (Tuik, 2016). 90% of cultivated plants in the world is grown with seeds. Use of healthy seeds is important for growing a healthy plant. A yield increase in plant production by 20-25%, and even more, can be achieved by using good quality of seeds. (Şehirali, 1989). As in most crops, the most important factors limiting productivity and quality in economically important vegetables are the diseases that many fungi cause are in the first rank and many of these diseases are also transported from field to field, and even from country to country, together with the seed (Şehirali, 1989). In order to use healthy seedlings in greenhouse growing areas, the used seeds should be healthy first. In our country many studies were conducted by different researchers in order to identify the fungal factors in some vegetable seeds. Various fungal factors were isolated in bean seeds in Erzurum (Demirci and Çağlar, 1998), and Eskişehir (Küçük et al., 2005) provinces and in some vegetable seeds (okra, pepper, tomato, spinach, zucchini, watermelon, melon, lettuce, leek and cucumber) in Konya (Er, 2010). Some fungal factors were identified in some plant seeds (wheat, barley,bean, corn, melon, cheakpea and leek) grown in the Lake Van basin (Demirer Durak et al., 2017). Seed borne pathogens lead to crop losses and decrease in germination performance of the seed, various physical and biochemical changes in seeds, toxine formation and decay of the seed (Neergaard, 1988). Most of these pathogens are fungal-based and they are on periphery, hull, endosperm and embryo of the seeds (Erkan, 1998). The seed, one of the most important production material in cultivation, needs to be free from diseases. Healthy seeds will both increase the yield and reduce the losses from diseases. In addition, reduction in the use of pesticides will also contribute to both producers economically and the health of human and environment. For this purpose, through this study conducted in 2016-2017 fungal flora will be identified in the seeds taken from several vegetable seeds (tomato, pepper, eggplant, cucumber, lettuce, parsley, arugula, cress, onion, spinach, cowpea, bean, pea and okra, etc.) grown in greenhouses in Central, Banaz, Esme and Sivaslı districts.
2. Material and Method
Material of the study consists of vegetable seeds of different species taken from Central district, Banaz, Esme and Sivaslı districts in which greenhouse vegetable growing is carried out. Economically important and commonly cultivated vegetable seeds (tomato, pepper, eggplant, cucumber, lettuce, parsley, arugula, cress, onion, spinach, cowpea, bean, pea and okra, etc.) were taken from naturally infected greenhouses in August and October. For isolation, used AP (AgarPlate) and DFB (Deep-Freezing Blotter) environments and fungal pathogens isolated from these environments formed the other materials of the study.
2.1. Agar Plate Method (AP)
Surface sterilized (S) and non-sterilized (NS) seeds were used in this method. For the surface sterilization, after the vegetable seeds were kept in 1% of NaOCl for 1 minute, they were washed 3 times with sterile distilled water, then dried between sterile drying papers. Surface sterilized and non-sterilized seeds were planted with equal distances according to each seed genus and size on PDA media containing streptomycin. Then, petri plates were left to incubation for 7 days at 22±2°C.
2.2. Deep Freezing Method (DFB)
Surface sterilized (S) (2 times dip into 1% of NaOCl for 3 minutes) and non-sterilized (NS) seeds were also used in DFB method. Then seeds were moisturized with sterile distilled water by putting in 9cm diameter of petri plates containing 3 layers of sterile filter papers with equal distances according to each seed genus and size. After petri plates were kept for 24 hours at 20°C, they were kept in deep freezer for 24 hours at -20°C. And then, they were left to incubation for 5 days under white flourescent light in 12 hours of light and 12 hours of darkness at 20 ± 2°C in climate room. For both methods, the experiments were carried out with 4 replicates and 25 seeds per replicate. Hyphe, mycelium and colony developments by fungal factors in seeds and feeding environment were examined through Deep-freezing (DFB) and Agar (PDA) methods recommended by Internation Seed Testing Association (ISTA) in order to diagnose the fungal microorganisms generating in vegetable seeds (ISTA, 1996; Mannerucci et al., 1982). For identification of the developing fungal factors at genus and species level, they were examined under x40 magnified light microscope and diagnosed according to the literatures. Fungal microorganism infection rate in seeds, the number of fungal microorganisms in examined seed and infected seeds were identified according to the indicated formula below.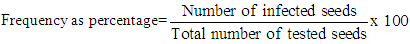
3. Results and Discussion
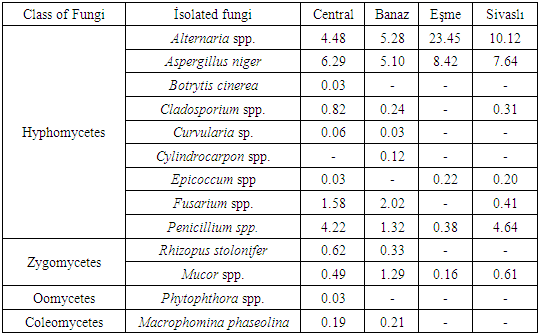
In this study, agar (PDA) and deep-freezing (DFB) methods were used in order to identify and diagnose the fungal factors in seed samples belonging to 14 different vegetables such as tomato, pepper, eggplant, cucumber, squash, lettuce, parsley, arugula, cress, spinach, bean, cowpea, pea and okra collected from the districts of Usak province where the greenhouse growing is carried out intensively.Aspergillus niger, Alternaria spp., Botrytis cinerea, Cladosporium spp., Curvularia spp., Cylindrocarpon sp., Fusarium spp., Epicoccum sp., Macrophomina phaseolina, Mucor spp., Penicillium spp., Phytophthora spp., Rhizoctonia spp. and Rhizopus stolonifer were identified in the tests conducted by using AP and DFB methods. Prevalance of the fungal factors isolated from vegetable seed samples collected from four districts in the study is indicated in Table 1. Fungal factors isolated from the vegetable seeds were mostly identified in Usak city center and then in Banaz. 13 species belonging to 4 fungi classes in total were isolated with both used methods. When the fungi isolated from the seeds were evaluated according to districts, whether surface disenfectation was carried out or not, Alternaria spp., Aspergillus niger, Penicillium spp. and Mucor spp. were identified in vegetables seeds taken from all districts as samples. In Sivaslı (% 10.12) and Esme (% 23.45) districts Alternaria spp. and Aspergillus niger (%7.64-8.42) were generally identified much in vegetable seeds. In all vegetable seeds Botrytis cinerea and Phytophthora spp. were identified only in central district, but Cylindrocarpon sp. was only identified in Banaz (Table 1).Table 1. Isolated fungi and occurrence percentage (%) in Uşak province at Central, Banaz, Eşme and Sivaslı location
 |
| |
|
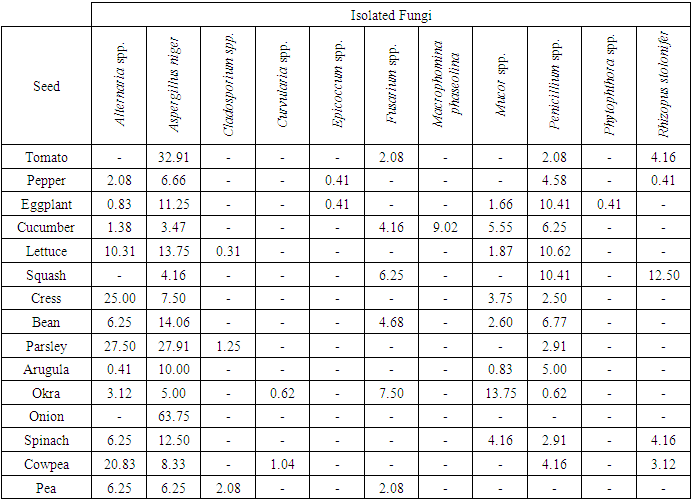
While fungus genus and species identified by AP method were 11, this was found as 10 by DFB method. It can be seen that the number of identified fungus genus and species was close to each other for both methods. However, presence of fungi rates varied in both two methods (Table 2 and 3). According to AP method, the most infected seeds with fungal factors were eggplants, cucumbers and okras, but the least infected one was onion. Aspergillus niger (% 100), Penicillium spp. (% 86.6) and Alternaria spp. (% 80) were isolated most with this method. In zucchini seeds Rhizopus stolonifer (%12.50), in parsley seeds Alternaria sp. (%27.50), in onion seeds Aspergillus niger (%63.75) were commonly isolated. In pea seeds Cladosporium sp. (% 2.08), in cowpea seeds Curvularia sp. (%1.04), in eggplant and pepper seeds Epicoccum sp. ise (% 0.41), in eggplant seeds Phytophthora sp. (% 0.41) were isolated at low rates. Macrophomina phaseolina (%9.02) was only identified in cucumber seeds and Mucor sp. (%13.75) and Fusarium sp. (%7.50) were identified in okra seeds (Table 2).Table 2. Isolated fungi from some vegetable seeds by agar plate (AP) method and occurrence percentage (%)
 |
| |
|
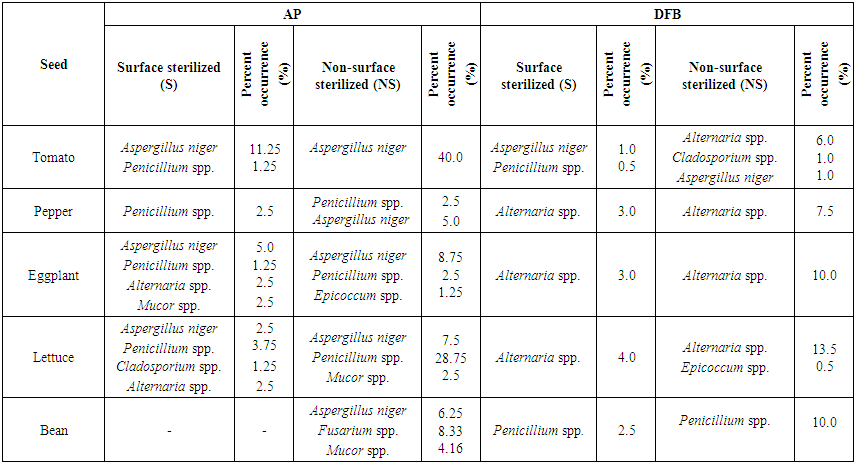
In Table 3, according to DFB method, the most infected seeds with fungal factor were lettuce and bean, the least infected seeds were pea seeds. With this method Alternaria spp. (% 86.6) and Fusarium spp. (%86.6), Penicillium spp. (% 80) and Aspergillus niger (% 60) were isolated most. With DFB method, in lettuce (%25.75) and parsley (%32.66) seeds Alternaria sp. marul (%25.75), in zucchini seeds Penicillium sp. (% 33.75), Aspergillus niger (% 68.75), Alternaria sp. (%16.25), in cowpea (%11.87) and pea seeds (%11.25).Fusarium spp. were commonly isolated. Also Alternaria spp. was identified at low rates in tomato and pepper seeds (% 5.33), eggplant (%7.16), cress (% 5.25) and okra (% 5) seeds. Cladosporium sp. was recorded in tomato, pepper, eggplant, lettuce, okra and spinach seeds at low (%0.12-1.33) rates. Botrytis sp. (%0.25) was only recorded in okra seeds, Curvularia sp. was only recorded in bean seeds and sadece Cylindrocarpon spp. was only recorded in cowpea seeds. However, Epicoccum spp. was identified in lettuce (% 0.12) and bean seeds (% 1.25) and Mucor spp. from saprophytic species, was only identified in squash seeds (Table 3).Table 3. The fungal microorganisms and occurrence percentage (%) in some vegetable seeds by the Deep-Freezing (DFB) method
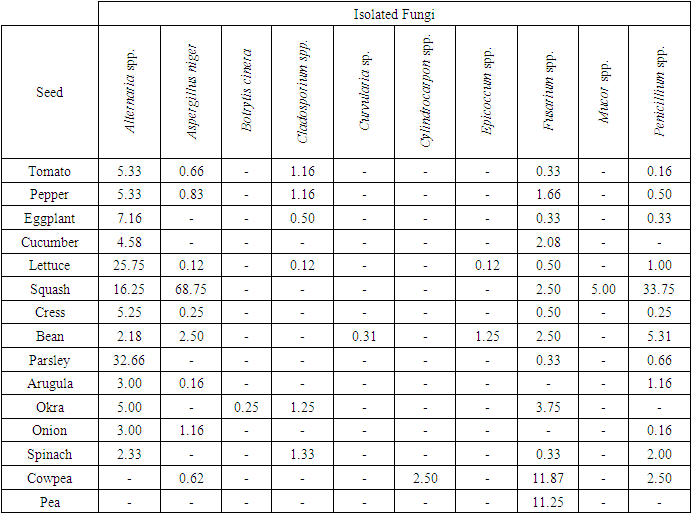 |
| |
|
In a conducted study Aspergillus spp., Penicillium spp. and Rhizopus stolonifer from the saprophytic species were especially observed in all vegetable seeds in blotter and agar methods. In blotter method it was observed that Aspergillus spp. among the saprophytic organisms was in watermelon seeds most and in leek seeds least; Penicillium spp. was in watermelon seeds most and in okra seeds least; Rhizopus stolonifer was in zucchini seeds most and in spinach seeds least. However, in agar method it was observed that Aspergillus spp. was in zucchini seeds most and in okra seeds least; Penicillium spp. was in tomato seeds most and in leek seeds least; Rhizopus stolonifer was in squash seeds most and in lettuce seeds least (Er, 2010).Vegetable seeds taken from each districts (Center, Banaz, Esme and Sivaslı), both AP and DFB methods and surface sterilized (S) and non-sterilized applications of these methods were evaluated in Table 4, 5, 6 and 7. Infection rates of seed samples in NS application of both AP and DFB methods were determined more than S application in general. In non-sterilized practice of AP method all of the seed samples taken from Central district were found to be infected with fungal factors. In sterilized application of AP method only spinach seeds were found as non-infected. Especially the saprophyte species Aspergillus niger., Penicillium spp. were observed in most of vegetable seed samples in AP methods (especially in NS practice) (Table 4, 5, 6, 7). In both two applications (S and NS) of both AP and DFB methods fungal infection in non-sterilized seeds were found more than sterilized seeds. No significant difference was found at genus and species level in terms of fungal factors among the methods.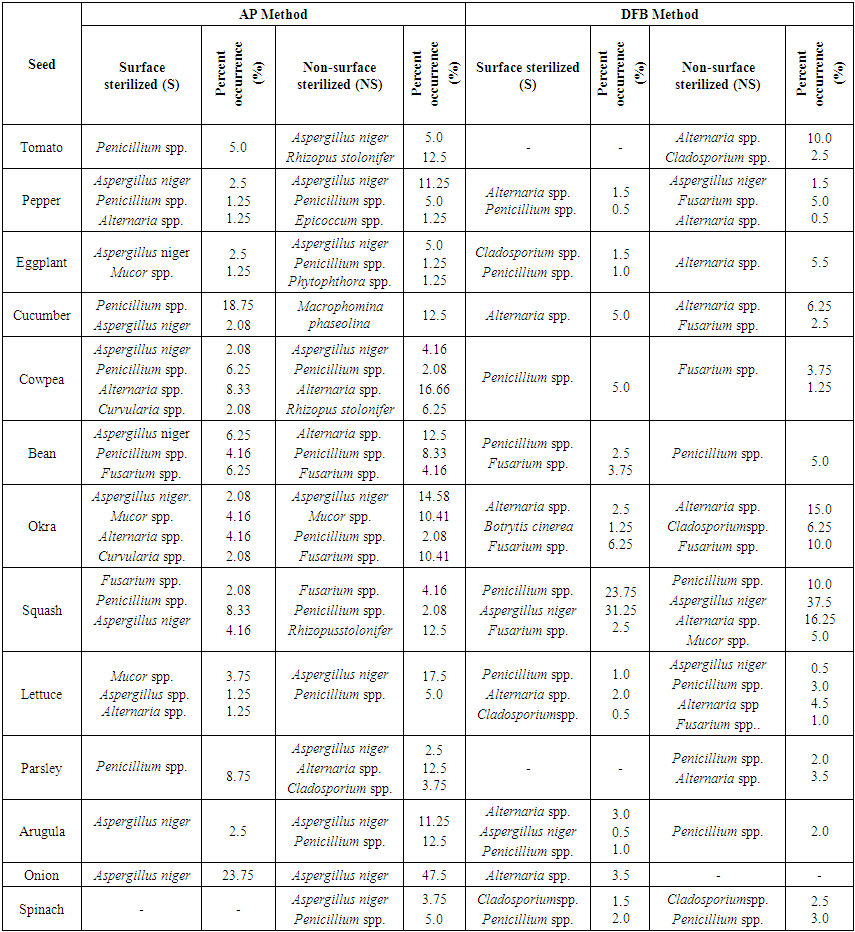 | Table 4. Fungal microorganisms and occurrence percentage (%) detected in vegetable seeds collected from central district |
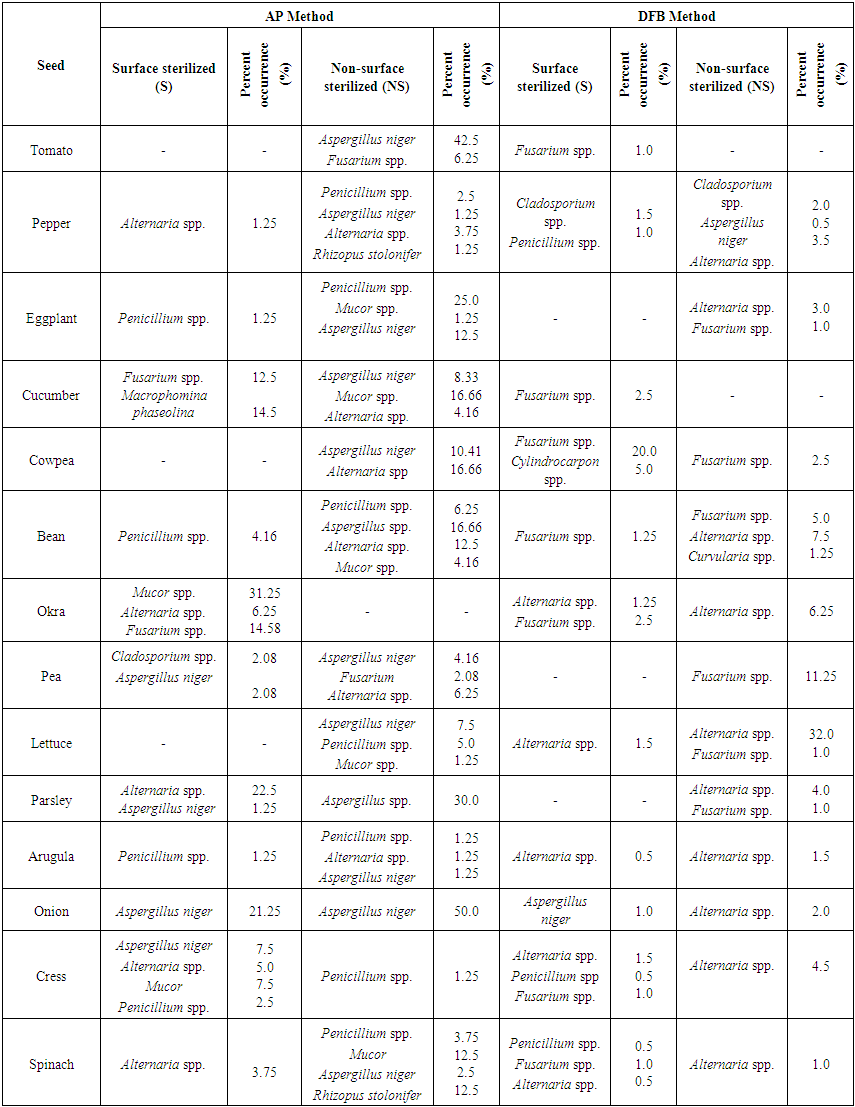 | Table 5. Fungal microorganisms and occurrence percentage (%) detected in vegetable seeds collected from Banaz district |
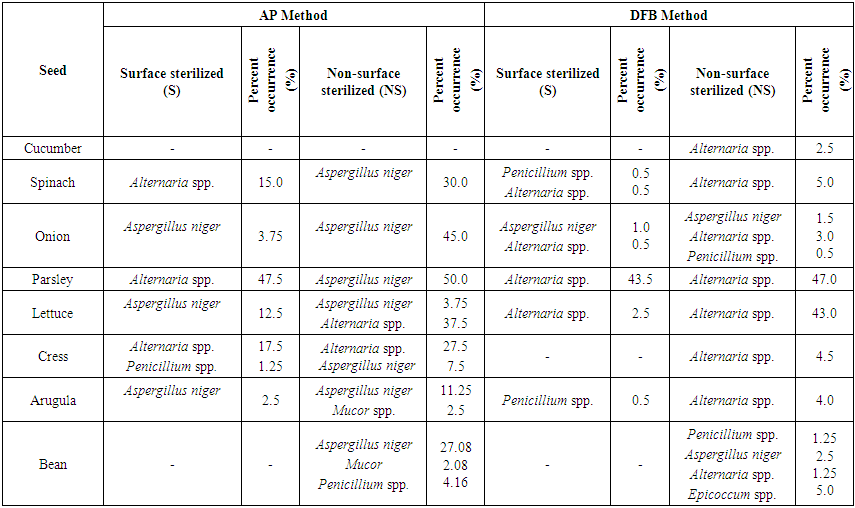 | Table 6. Fungal microorganisms and occurrence percentage (%) detected in vegetable seeds collected from Eşme district |
 | Table 7. Fungal microorganisms and occurrence percentage (%) detected in vegetable seeds collected from Sivaslı district |
In Table 4, the fungus which presents most in NS and S applications of AP method in all seeds in Usak province center was Aspergillus niger (% 76.92). It was recorded that the least infected seeds were lettuce (% 1.25) seeds, the most infected seeds were onion (% 23.75) seeds in NS application. However, this rate was identified in parsley (% 2.5) seeds as the least and in onion (% 47.5) seeds as the most in S application. Penicillium spp. was identified in approxiamately 54% of the seed samples taken from Central distirict in S application (in AP method), but it was found 69% in NS application (Table 4). In NS application Rhizopus stolonifer was identified in tomato, zucchini and cowpea seeds (% 6.25) and Mucor spp. was identified in okra (% 10.41) seeds (Table 4). In cucumber seeds Macrophomina phaseolina (%12.5), one of the important species, in pepper, cowpea, bean, okra, parsley and lettuce seeds Alternaria spp. (% 1.25-16.66) were isolated in AP method (without regarding S and NS applications). In squash, okra and bean seeds Fusarium spp. (%2.08-10.41) and in okra and black-eyed bea seeds Curvularia spp. were identified evenly. In only parsley seeds Cladosporium spp. and in only eggplant seeds Phytophthora spp. were observed. Agar Plate was a convenient method in order to diagnose the surface infections of slowly growing fungi. However, disadvantage of this method is that slowly growing fungi or the fungi inside the seeds are pressurized by other fast growing fungi (Neergaard, 1988). Most of fungi species which were found to be on the surface of seeds decreased in number with the surface sterilization. Fungi density identified by Blotter method at different parts of the seed was determined to be more than AP and DFB method. (Dawar et al., 2007). In only tomato, lettuce, spinach, eggplant and okra seeds Cladosporium spp. and in 80% (% 0.5-16.25) of vegetable seeds Alternaria spp. were observed in DFB method (without regarding N and NS applications). However, Botrytis cinerea was isolated only in okra seeds (Table 4).In Table 5 it was recorded that in NS application of AP method in Banaz approximately 86% of the vegetable seeds was infected with Aspergillus niger and 50% of them was infected with Penicillium spp. However, Mucor spp. was identified in approximately 36% of the vegetable seeds and the infection rate was identified less. The most infection rate with Aspergillus niger was observed in onion (50%), tomato (42.5%), parsley (30%) and bean (16.66%) seeds. Respectively (in S application) in parsley, okra, cress, spinach and pepper seeds Alternaria spp. (% 1.25-22.5), in okra seeds Fusarium spp. (% 14.58) and in pea seeds at low rates Cladosporium spp. (% 2.08) were isolated in AP method. However, in cucumber seeds Fusarium spp. (% 12.5) and Macrophomina phaseolina (% 14.5) were identified. However, in cucumber seeds Fusarium spp. (% 12.5) and Macrophomina phaseolina (%14.5) were identified (Table 5). In lettuce, arugula, cress and spinach seeds Alternaria spp., in cowpea and bean seeds Fusarium spp. and in pepper seeds Cladosporium spp. were the dominant fungal factors in DFB method (in S and NS applications). Also Cylindrocapon sp. (cowpea) and Curvularia spp. (bean) were recorded less only in two vegetable species (Table 5). In another conducted study Alternaria spp., Fusarium spp. and Aspergillus spp. were the most identified fungi in okra and tomato seeds (Al-Kassim et al, 2000). Again in a different study it was reported that cucumber seeds isolated Macrophomina phaseolina from embryo and testa cotyledon parts (Sultana et al., 2009).In seed samples taken from Esme and Sivaslı more Aspergillus niger., Penicillium spp. were isolated in both applications (S and NS) of AP method than DFB method (Table 6,7). Aspergillus niger was respectively identified most in parsley (50%), onion (45%), spinach (30%), bean (27.08), arugula (11.25%), cress (7.5%) and lettuce (3.75%) seeds in NS application (AP method). AP technique identifies the seed borne saprophytes effectively. This derives from the stimulating factors on potato dextrose agar. In addition, AP method provided for the recovery of some fungi not found in DFB method. This may be due to the need for fungi to get nutrients that are not found in seeds outside (Panchal and Dhale, 2011).In addition, while parsley seeds were infected with 47.5% of Alternaria spp. only in S application of AP method, they were found infected with 43.5-47% of rates in both applications of DFB method. Furthermore, cress seeds were infected with Alternaria spp. (% 17.5-27.5) in S and NS applications of AP method, lettuce seeds were identified to be infected (% 2.5 ve % 43) in both applications (S and NS) of DFB method. Mucor spp. among different genus (in AP method) was recorded less in arugula (2.5%) and bean (2.08%) seeds and Epicoccum spp. was recorded less only in bean seeds (5%) (Table 6). In vegetable samples in Sivaslı commonly identified fungi were Aspergillus niger, Penicillium spp. and Alternaria spp. In both applications (S and NS) of AP method Aspergillus niger was identified much (% 2.5- 11.25- % 5- 40), however, Penicillium spp. was identified less but in most of seed samples. It was only identified much (% 28.75) in lettuce seeds in NS applications. Among the different genus Epicoccum spp. was only recorded in eggplant seeds (% 1.25) and Fusarium spp. was recorded in bean seeds (% 8.33). However, in DFB method the same fungal factors were isolated in both two applications (S and NS). In S application in pepper, eggplant and lettuce seeds Alternaria spp.;however, in NS application in only tomato seeds Cladosporium spp. and in bean seeds Epicoccum spp. were isolated (Table 7).According to districts of the distribution of isolated fungi from all vegetable seeds, it was identified that Alternaria spp., Aspergillus niger, Botrytis cinerea, Cladosporium spp., Curvularia spp., Cylindrocarpon sp., F.equiseti, Penicillium spp., R.stolonifer and S.botryosum existed in various rates in seeds depending on surface disinfectation applications according to AP and DFB methods in all districts that the samples were taken. The fungi apart from this ones were isolated in different rates from the seeds taken from some districts. In some conducted studies some soil-based fungi were reported in tomato seeds in different countries (Mathur and Manandhar, 2003). In a study in Saudi Arabia it was reported that seed borne fungi in tomatoes limited the production. In tomatoes Alternaria alternata, Botrytis cinerea, C. herbarum, Drechslera sp., F. oxysporum, P. aphanidermatum, R. solani and V. Alboatrum were reported as seed borne fungi (Al-Kassim and Monawar, 2000). In Agar plate (AP) method Aspergillus flavus, A. niger, Aureobasidium pullulans, Geotrichum candidum, Penicillium polonicum and Rhizopus stolonifer; however, in Deep-freezing (DFB) method Alternaria alternata, Cladosporium spp., Stemphylium botryosum, Ulocladium alternaria were commonly found in a study on tomato seeds by different researchers in Saudi Arabia (Al-Askar et al., 2014). Some studies concerning vegetable seeds were conducted in our country, but these studies were conducted with seed samples taken from open-field grown vegetable and cultivated plants. In a study with bean seed samples Demirci and Çağlar (1998) identified that they were infected with Alternaria alternata, Aspergillus spp., Botrytis cinerea, Cladosporium spp., Colletotrichum lindemuthianum, Fusarium acuminatum, F. equiseti, F. proliferatum, F. verticillioides, Penicillium spp., Phoma glomerata, P. medicaginis, Rhizoctonia solani, Rhizopus stolonifer, Stemphylium botryosum, Trichoderma spp., Trichothecium roseum and Ulocladium atrum. In studies by different researchers the most common fungi were identified as Pythium spp. (%77.24) in okra seeds, Sclerotinia spp. (%46.39) in pepper seeds, Botrytis cinerea (%34.81) in tomato seeds, Fusarium solani (%30.55) in cucumber seeds, Fusarium culmorum (%40.86) in spinach seeds, Fusarium solani (%30.36) in zucchini seeds and Botrytis cinerea (%43.63) in lettuce seeds (Er, 2010). In a recent study fungal factors were identified through AP method using the seeds of wheat, barley, bean, corn, chickpea and leek cultivated in Lake Van basin. As a result of conducted isolations it was identified that seed samples were generally contaminated with Penicillium spp., Fusarium spp. and Alternaria spp. Especially Fusarium graminearum was intensively isolated from wheat and barley, Fusarium oxysporum was isolated from melon, leek and corn and Macrophomina phaseolina was isolated from bean and okra. (Demirer Durak et al.; 2017). In a study on pepper seeds Colletotrichum capsici (%54.75), Aspergillus niger (%44.00) and A. flavus (%29.75) were the most isolated fungi (Chigoziri and Ekefan, 2013).As a result, greenhouse growing is in a significant development process in our province where mostly dry agriculture is carried out. In order to get more yield from unit area producers prefer producing their own seedlings. This leads the disease factors to be transported from seed plots to greenhouse areas. In greenhouse vegetable growing developing in Usak province surveys should be carried out in order to research and prevent the yield losses from disease factors transported with seeds and producers should be informed about the importance of this issue.
References
| [1] | Abak, K. 2012. Türkiye’de Sebze Tarımının Kırk Yılı. 9. Ulusal Sebze Tarımı Sempozyumu.Konya 1-7. |
| [2] | Al-Kassim, M.Y., and Monawar, M.N. 2000. Seed-borne fungi of some vegetable seeds in Gazan province and their chemical control. Saudi J Biol Sci 7: 179–184. |
| [3] | Al-Askar, A.A., Ghonem, K.M., Rashad, Y.M., Abdulkhair, W.M., Hafez, E.E., Shabana, Y.M., Baka, Z.A., 2014. Occurence and Distribution Of Tomato Seed-Borne Mycoflora İn Saudi Arabia and İts Correlation With The Climatic Variables. Microbial Biotechnology (2014) 7(6), 556–569 doi:10.1111/1751-7915.12137. |
| [4] | Chizogori, E., and Ekefan, E.J. 2013. Seed borne fungi of Chilli Pepper (Capsicumfrutescens) from pepper producing areas of BenueState, Nigeria. Agrıculture and Bıology Journal of North America. |
| [5] | Dawar, S., Syed, F. and Ghaffar, A. 2007. Seed Borne Associated with Chickpea in Pakistan. Pak. J. Bot., 39(2): 637-643, 2007. |
| [6] | Demirci, E., Çağlar, A., 1998. Erzurum İlinde Fasulye Tohumlarından İzole Edilen Funguslar. Bitki Koruma Bülteni 38, (1-2): 91-97. |
| [7] | Demirer Durak, E., Bilici, S. and Günaydın, Ş. 2017. Van Gölü Havzası’nda Yetiştiriciliği Yapılan Bazı Bitki Tohumlarında Elde Edilen Funguslar ile Patojeniteleri. Turkish Journal of Science, Volume II, Issue I, 7-14. |
| [8] | Er, Y., 2010. Bazı Sebze Tohumlarında Fungal Floranın Tespiti ve Tanılaması. Yüksek Lisans Tezi. Selçuk Üniversitesi Fen Bilimleri Enstitüsü Bitki Koruma Ana Bilim Dalı. Konya. |
| [9] | Erkan, S., 1998. Tohum Patolojisi. Gözdem Ofis, Izmir, 275 s. |
| [10] | ISTA, 1996. International Rules for Seed Testing, Annexes 1996. Seed Sci and Technol. 24 (Suppl.): 13-19, 93-122, 247-252. |
| [11] | Küçük, Ç., Kıvanç, M., Çakır, S., ve Hasenekoğlu, İ., 2005. Eskişehir İlinde Kuru Fasulye Tohumlarından İzole Edilen Funguslar. Orlab On-Line Mikrobiyoloji Dergisi. 03, 1-4. |
| [12] | Mathur, S.B., and Kongsdal, O. 2003. Common Laboratory Seed Health Testing Methods for Detecting Fungi, 1st edn. Bassersdorf, Switzerland: International Seed Testing Association. |
| [13] | Mathur, S.B., and Manandhar, H.K. 2003. Fungi in Seeds: Recorded at the Danish Government Institute of Seed Pathology for Developing Countries. Copenhagen, Denmark. |
| [14] | Neergaard, P., 1988. Seed Pathology Vols. I and II, MacMillan Press, Hong Kong, XXV+ 1191 p. |
| [15] | Mannerucci, F., Gambogi, G., and Vannaccı, P.1982. A survey of pathogenıc fungi detected in vegetable seeds. |
| [16] | Sultana, N. and Ghaffar, A. 2009. Seed-borne Fungi Associated with Bottle Gourd. Pakistan. Journal of Botany, 41(1): 435-442, 2009. |
| [17] | Panchal, V.H., and Dhale, D.A. 2011. Isolation of seed-borne fungi of sorghum (Sorghum vulgare pers.). J Phytol 3: 45–48. |
| [18] | Şehirali, S., 1989. Tohumluk ve Teknolojisi. A.İ.Z.F. Basımevi, Ankara, XII+330 s. |
| [19] | Tuik, 2016. Türkiye İstatistik Kurumu. www.tuik.gov.tr. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML

