-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(2): 53-59
doi:10.5923/j.ijaf.20180802.01

Insecticidal Activities of Phyllanthus emblica, Prunus mahaleb, Cerasus mahaleb, Piper nigrum, Krameria lappacea and Phoenix dactylifera on Larvae Trogoderma granarium Everts
N. Al-Fuhaid
Department of Biology, Sattam Bin Abdul-Aziz University, College of Science and Humanities, Kharj, Saudi Arabia
Correspondence to: N. Al-Fuhaid, Department of Biology, Sattam Bin Abdul-Aziz University, College of Science and Humanities, Kharj, Saudi Arabia.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

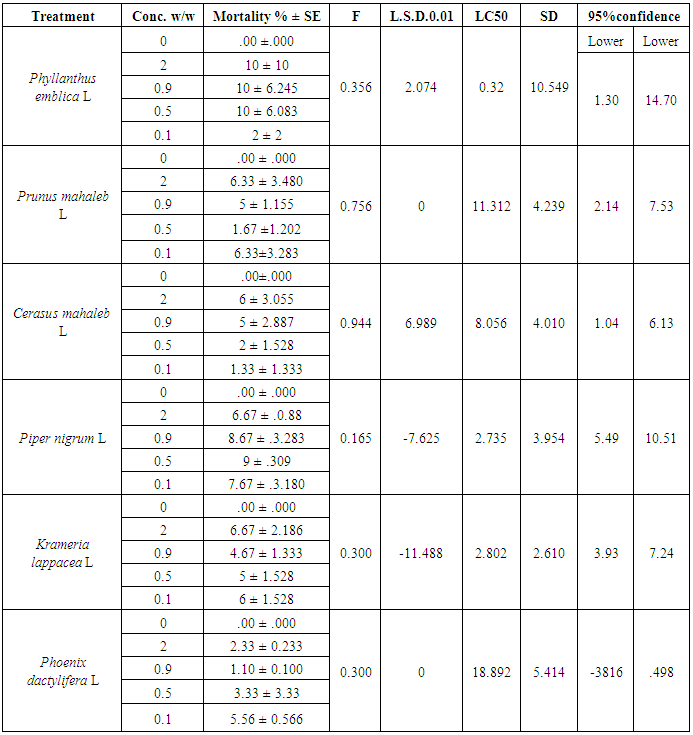
The repellency and toxic activity of some plants that are commonly used in folk medicine are evaluate against the destructive 5th instar larvae of the Trogoderma granarium beetle. Wheat grains were treated with selected powders of Phyllanthus emblica, Prunus mahaleb, Cerasus mahaleb, Piper nigrum, Krameria lappacea and Phoenix dactylifera at concentrations of 2, 0.9, 0.5, or 0.1% for 72 hours. The K. lappacea, P. emblica and P. mahaleb powders provided 53, 53 and 45% repellency respectively at 2% after 72 h; the other powders showed only medium to weak repellency. On the basis of LC50, the tested toxicity effectiveness can be arranged as P. emblica (0.32%) > P. nigrum (2.7%) > K. lappacea (2.8%) at ten days. Notably, the powders of C. mahaleb, and P. dactylifera resulted in a strong attraction of larvae to the treated grains at -100% at any time for any tested concentration. The repellent and toxic effects of P. emblica fruit powder are apparent. Powders of P. nigrum, K. lappacea and P. emblica can be considered promising environmentally healthy and safe pesticides to reduce the loss of stored wheat grains caused by this larvae eater.
Keywords: Piper Nigrum, Krameria Lappacea, Secondary Metabolism, Proanthocyanidin Tannins, Physical Toxicity
Cite this paper: N. Al-Fuhaid, Insecticidal Activities of Phyllanthus emblica, Prunus mahaleb, Cerasus mahaleb, Piper nigrum, Krameria lappacea and Phoenix dactylifera on Larvae Trogoderma granarium Everts, International Journal of Agriculture and Forestry, Vol. 8 No. 2, 2018, pp. 53-59. doi: 10.5923/j.ijaf.20180802.01.
Article Outline
1. Introduction
- The beetle Trogoderma granarium Everts (Coleoptera: Dermestidae) is considered one of the most destructive pests of various types of stored grains, especially wheat, and can significantly damage the grain germs [1]. They are mainly found in India, other tropical and subtropical countries, Mediterranean countries, Iraq, and Iran. Its presence has also been recorded in southern Europe, western Africa, Asia and South America [2]. It is characterized by being able to withstand various environmental conditions [3, 4].The difficulty of controlling this insect lies in its habitation with foodstuffs and the fact that the use of pesticides leads to the emergence of resistance to the action of pesticides [5, 6]. Furthermore, the use of methyl bromide gas as a pesticide is dangerous and carcinogenic to humans and causes genetic mutations in embryos [7-9]. Therefore, the attention and efforts of researchers have turned towards finding safe alternatives to protect the environment and human health and the wholesomeness of food. The most prominent of these alternatives was the use of plants and their parts, extracts and oils to control harmful insects in their various stages, both in the field and in storage [10-12].In fact, the search for effective plants to repel or kill field or storage pests was started by primitive farmers and housewives [13-16]. Such simple attempts, especially in rural communities, whether effective or not, have played an important role in the creation of these alternatives, beginning with the development of some of them into herbicides based on compounds separated from plants and commercialized; the most famous are Azadirachta indica neem [17-19] Derris elliptica (Wall.) Benth rotenone [20, 21] and Chrysanthemum cinerariifolium Pyrethrum [22, 23]. Due to the huge losses in quality and quantity and the economic damage to stored wheat grains caused by Trogoderma granarium larvae, a search for alternative controls is imperative [24]. The most important larval stage of the Trogoderma granarium beetle that destroys stored wheat grains is the fifth instar larvae [25]. This study was conducted to test the repellent and toxic effects of selected parts from some plants that are commonly used in traditional and alternative medicine in Asia and Saudi Arabia, namely, Prunus mahaleb, Cerasus mahaleb, Piper nigrum, Krameria lappacea and Phoenix dactylifera, against the fifth-instar larvae of Trogoderma granarium.
2. Materials and Methods
2.1. Breeding the Insect
- Adults and larvae of Trogoderma granarium were collected from wheat grains Triticum aestivum and were cultured in an incubator at a temperature of 26±2°C and relative humidity of 70-60% in the laboratories of the Department of Biology, Faculty of Science and Humanities at Sattam bin Abdul Aziz University. First, 200 g of sterile wheat was placed in 20-cm-long and 12-cm-diameter containers. The containers were covered with a muslin cloth with a rubber band attached and incubated in the incubator. The culture was constantly renewed after each generation.
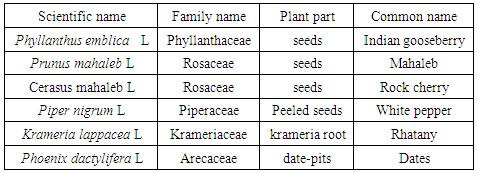
2.2. Plant Materials
- The tested plants and their parts are shown in Table 1. Phyllanthus emblica, Prunus mahaleb, Cerasus mahaleb, Piper nigrum, Krameria lappacea and Phoenix dactylifera were purchased from local market, analysed by a specialized taxonomist, cleaned of dust and particles, washed, air-dried and finally ground by a high-voltage electric mill, sifted through a 60 mesh sieve and kept in refrigerators until use.
|
2.3. Bioassay
2.3.1. Repelling Effect of the Plant Powders
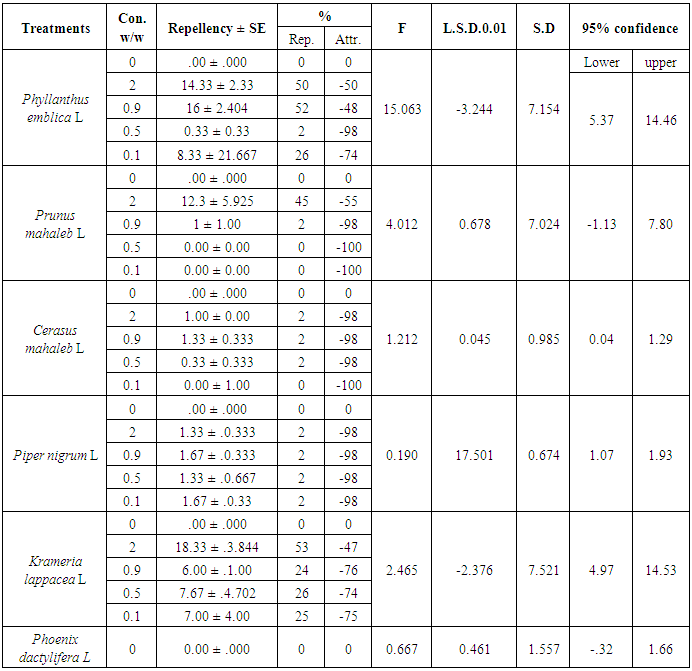
- In accordance with the methodology given in [26] and revised by [27], glass dishes that were 11 cm in diameter and 2 cm in height were used. Each dish was divided into two equal halves, and a 2-cm-diameter circle was drawn in the centre of one of the halves. The plant powder at a given concentration was placed on one half of the paper, and the other half was free of any treatment. Then, 10 larvae 5th instar was placed in the test area (centre of the paper). The repellency percentage was measured after 15 and 30 minutes and after 24, 48 and 72 hours, and a perforated plastic lid were placed on the glass dish. The number of insects was calculated in the non-treated half (C), with 3 replicates per tested plant powder for specific concentrations (2, 0.9, 0.5 and 0.1%). The repellency percentage was calculated using the following equation:PR = 2(C-50%)PR = percentage of repellencyC = percentage of insects in non-treated halfIf C is more than 50%, PR becomes positive, and the treatment by plant powder has a repellent efficiency. Conversely, if C is less than 50%, PR becomes negative, and the plant powder has an attractive effect.
2.3.2. Effect of Plant Powders on Percentage of the 5th-Instar Larval Mortality
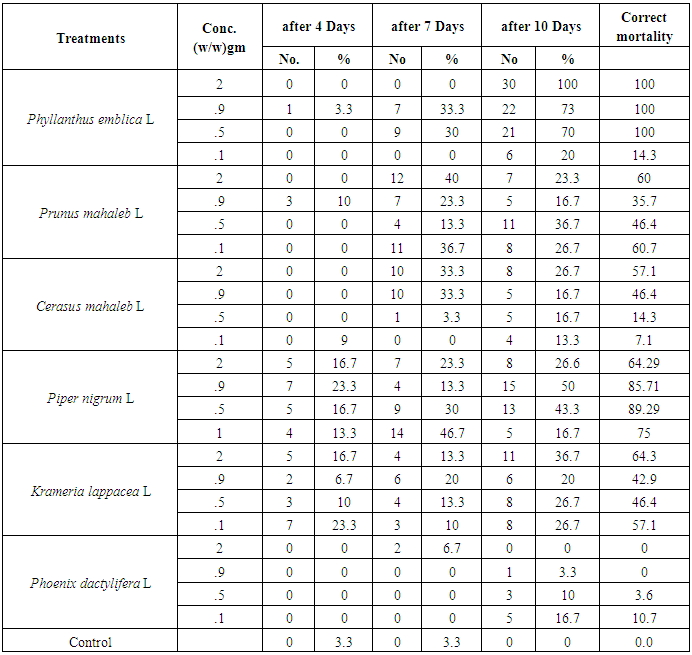
- Various concentrations of plant powders (2, 0.9, 0.5 and 0.1%) were added to the sterile wheat grains, kept at 60°C in the electric oven for 2 hours and placed in sterilized Petri dishes. Then, 100 wheat grains treated with specific concentrations were placed in each dish, and 10 newly moulted 5th-instar larvae were added, with three replicates. In the control treatment, the 5th-instar larvae were fed untreated wheat grains, and the percentage of mortality was reported after seven days of treatment.
2.4. Statistical Analysis
- The mortality data were corrected with control mortality following the formula given in Abbot [28]. All data were statistically analysed using a one-way analysis of variance (ANOVA, SPSS statistical analysis software, version 11) [29]. The means were compared using the least significant difference (L.S.D) at the 0.10 level.
3. Results
3.1. Repellent or Attractive Effect of the Plant Powders
- The results provided in Table 2 show the repellent effects of the tested plant powders. The K. lappacea, P. emblica and P. mahaleb powders provided 53, 52 and 45% repellency respectively at 2% after 72 h Conversely the attractive percentage is -47, -48 and -55% for K. lappacea, P. mahaleb and P. emblica respectively at 2% after 72 h. It should be noted that low concentrations (0.1 and 0.5%) of both P. emblica and K. Lappacea increased in repellent effectiveness as the exposure time increased. However, the repellency percentage was only 2% for treatments with 2% of plant powders of P. nigrum and C. mahaleb. The powder of P. dactylifera did not provide repellency against the larvae exposed to it at any time for any tested concentration but have a strong attractive effect for 5th treated larvae at -100% attractive percentages at all tested at any time.
|
3.2. Effect of Plant Powders on 5th Instar Larval Mortality
- The effect of the plant powders was tested using the percentage of 5th-instar larvae that died. The results in Table 3 show a 100% increase in the percentage of mortality when wheat grain was treated with the P. emblica powder at concentrations of 2, 0.9 and 0.5% after ten days of treatment. P. nigrum powder at concentrations of 0.5 and 0.9 g resulted in mortality percentages of 85.7% and 89.3% after 10 days of treatment compared to the values of 60%, 64% and 57% that were found for the highest concentration (2%) of the three plant powders P. mahaleb, K lappacea and C. Mahaleb , respectively, after 10 days of treatment.
|
|
4. Discussion
- The results show that the repellency and toxicity of the different plant powders varied in effectiveness, even if are not significantly different at the 0.01 level of probability, which may have been related to differences in the chemical contents of the plant powders tested.The powders of K. lappacea, P.emblica and P. mahaleb each demonstrated a strong positive relationship between repellency percentage and exposure time.In contrast, a highly toxic effect was seen only in P. emblica, P. nigrum and P. mahaleb powders. It is worth noting the varying effectiveness of the K. lappacea and P. nigrum powders with regard to repellency and toxicity effects, respectively. The variation of the interactions suggested that the different biological activities of the chemical contents of the tested plants may be a result of a variation in the tested parts and their use in the test as a powder, aqueous or alcoholic extract [12, 30-32].It should be noted that the fruit powders of P. emblica and P. mahaleb retained the effects of repellency and toxicity on the larvae exposed to treated food. To explain the mechanism underlying the potential toxicity effects of the applied treatments, although a repellency effect was demonstrated for the 5th-instar treated larvae, the behavioural indicators of the repellency effect were also observed for escaped larvae that were far from the treated wheat grains. This dual effect is due to the physical and chemical properties of some plants that possess general larvicidal activity. This result is close to that observed by Ivbijaro [33], who determined some physical and chemical toxicity requirements for plants to be considered to have larvicidal activity. Additionally, some possible explanations for our results are as follows:Additionally, it is possible the powder particles from treated wheat grains closed the spiracles and prevented the larvae from obtaining oxygen [34]. Considering the weak movement of the treated larvae compared to untreated ones, and their movement towards the bottom of the experiment dish, under the treated grains, this behaviour may preliminarily indicate diapause due to fasting from the inappropriate food available. This hypothesis agrees with feeding deterrence effects caused by treatment with other plant powders [35, 36]. The adhesion of plant powder on the body wall of treated larvae can have harmful chemical effects on some physiological functions. The most prominent of these changes are the scraping of the wax layer of the insect’s cuticle and the resulting loss of water, thus exposing the insect to drying and potential mortality [37-39] and the possibility of absorption of cuticle fat, especially in some of the tested plants. K. lappacea, P. emblica, and P. mahaleb are characterized by high contents of tanning compounds, which are known [40, 41] for their ability to drain the surfaces on which they rest due to their astringent properties; these plants in particular have proanthocyanidin tannins [42] and are used to treat burns and stitching wounds [43].The diversity in repellency and toxic activity among the tested plants is due to the diversity of their chemical content. Therefore, some of these plants may be accepted by some insects. It is clear that seed powder of C. mahaleb and date-pits of P. dactylifera show this kind of acceptance, as they were not effective in the repellency and toxicity tests. However, Abushaala et al. [44] proved that a date-pits extract of P. dactylifera had anti-fungal activity at a concentration of 15. According to [45] P. dactylifera date-pits contain moisture, protein and carbohydrate contents of 12.5, 6.9 and 86.9 g/100 g date-pits, respectively. This nutritional content may explain the main reason why the larvae accepted wheat grains treated with powder from the date-pits.Samuel et al. [46] demonstrated the larvicidal effect of piperine alkaloid on the 4th-instar larvae of mosquitoes in the Anopheles Malaria Complex Strains by feeding the larvae with a dose of piperine. In that study, a dose of 10% of pepper powder resulted in more than 70% mortality. Additionally.Adnan et al. [47] and Hasan et al. [48] reported P. emblica to have many bioactive compounds that are important in medicinal uses, including anti-bacterial, anti-oxidant, and anti-inflammatory compounds. The effectiveness of P. emblica may be due to the plant's content of different levels of terpenoids, ascorbic acids and secondary metabolites, such as tannins. K. lappacea roots are popular medical treatments for burns, wounds and dental care and were used 200 years ago in Europe in pharmacology [49, 50]. The main chemical content of root extracts is tannin compounds, which are effective in combating microbes and antioxidants [51, 52]. In a previous literature review, it is clear that tannin compounds are available in the three plants K. lappacea, P. emblica, and P. mahaleb, which demonstrated repellency and toxicity against T. granarium 5th instar larvae. Tannin compounds are also indicated by the potent odour of the piperine compound in P. nigrum and the burning effect of direct contact [53- 56], two characteristics that are likely to play a role in the toxic and repellent activity on treated larvae.
5. Conclusions
- K. lappacea, P. emblica, P. nigrum and P. mahaleb plant powders were possess some of the biological activities compounds can be used to treat wheat grains and protect them from infection Trogoderma granarium and its larvae phases.
ACKNOWLEDGEMENTS
- I would like to thank M. Al-rwais, A. Al-Dosari, S. Qahtani and, and M. Al-Dosari for technical assistance from the Prince Sattam Bin Abdul-Aziz University, the College of Science and Humanities, and the Department of Biology for their invaluable help in completing this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML