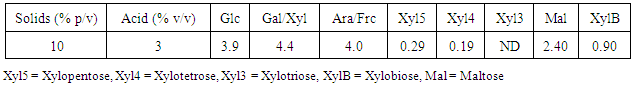-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(1): 26-34
doi:10.5923/j.ijaf.20180801.05

Production of Ethanol by Three Yeasts in Defined Media and Hydrolyzed Cladodes of Opuntia ficus-indica var. Atlixco
Rogelio Pérez-Cadena1, Teodoro Espinosa Solares2, Sergio Alejandro Medina-Moreno1, Alfredo Martínez3, Manuel Alejandro Lizardi-Jiménez4, Alejandro Téllez-Jurado1
1Biotechnology Department, Universidad Politécnica de Pachuca, Zempoala, Hidalgo, México
2Industrial Engineerig Department, Universidad Autónoma Chapingo, Texcoco, Estado de México, México
3Instituto de Biotecnología-UNAM, Cuernavaca, Morelos, México
4CONACYT-Instituto Tecnológico Superior de Tierra Blanca, Tierra Blanca, Veracruz, México
Correspondence to: Alejandro Téllez-Jurado, Biotechnology Department, Universidad Politécnica de Pachuca, Zempoala, Hidalgo, México.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Three yeast strains of Opuntia ficus indica cladodes were isolated, these strains were identified as Candida intermedia, Zygosaccharomyces bailii and Saccharomyces paradoxus. The yeasts were grown in synthetic media using as carbon source the main sugars detected in the acid hydrolysates of the cladodes: fructose, galactose, glucose and mannose. Different sugar consumption patterns were observed for each of the yeasts under study, being the main sugars consumed; glucose and fructose. Under these conditions, Z. bailii showed the highest biomass production with a growth rate of 0.146 h-1. However, C. intermedia was the yeast with the highest ethanol yield with 0.299 g of ethanol/g of biomass. When the ethanol production was evaluated in co-fermentation with the three strains, an increase in ethanol production of 300% (0.727 g of ethanol/g of biomass) was observed with a conversion efficiency of 28.9%. Subsequently, the Opuntia hydrolyzate was used as the base of ethanol production medium, the yeast that showed the best behaviors was C. intermedia with yields of 0.321 g of ethanol/g of biomass and conversion efficiency of 63.029%.
Keywords: Cladodes, Hydrolysis, Opuntia ficus-indica, Yeast
Cite this paper: Rogelio Pérez-Cadena, Teodoro Espinosa Solares, Sergio Alejandro Medina-Moreno, Alfredo Martínez, Manuel Alejandro Lizardi-Jiménez, Alejandro Téllez-Jurado, Production of Ethanol by Three Yeasts in Defined Media and Hydrolyzed Cladodes of Opuntia ficus-indica var. Atlixco, International Journal of Agriculture and Forestry, Vol. 8 No. 1, 2018, pp. 26-34. doi: 10.5923/j.ijaf.20180801.05.
Article Outline
1. Introduction
- The genus Opuntia in Mexico is cultivated in 29 federative entities that produce about 723,000 tons in 12,500 hectares. For 2010 the area increased 5 times and the production of forage nopal was 208,492 tons [1]. Although its chemical composition is variable, the presence of mucilage and fibers are the main components that constitute the cladodes of Opuntia sp. The fibers is composed of a bilateral network of lignocellulosic residues that with age harden and provide a rigid constitution, increasing the thickness and intensifying the colour [2]. The lignocellulosic residues contain cellulose
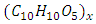 and hemicellulose
and hemicellulose 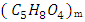 which are bound to the lignin
which are bound to the lignin 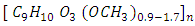 [3]. Since the conversion of cellulose and hemicellulose into sugar monomers such as 5 and 6 carbon carbohydrates is complicated, one of the main aspects to consider in the use of lignocellulosic residues for the production of bioethanol is the pre-treatment to destroy the cellulose matrix and dispose of fermentable sugars [4].Starting from the pretreatment or treatment of the lignocellulosic material, sugars such as hexoses are obtained, which can be efficiently converted to bioethanol with yields between 0.4 and 0.5 g/g with productivities greater than 1 g/L h when using Saccharomyces cerevisiae. Similar productivities can be obtained from mesophilic microorganisms such as Escherichia coli, Klebsiella oxytoca y Zymomonas mobilis Clostridium sporogenes, C. indolicus, C. sphnoides, Erwinia amilovora, Spirocheta aurantia, Streptococus lactis, Spirocheta litorales and Spirocheta stenostrepta [5]. However, S. cerevisiae is unable to ferment pentoses derived from hemicellulose such as xylose and arabinose [6]. The xylose is the most predominant pentose in hemicellulose, in addition, arabinose can constitute from 2-20% of the sugars in the hemicellulose depending on the agricultural residue in question [3]. Therefore, due to the high number of pentoses present in the lignocellulosic biomass, it is important to make efficient the use of these sugars by microorganisms to reduce production costs [6].Among the microorganisms that are able to naturally degrade pentoses such as xylose, are some yeasts such as Candida, Kluiveromyces, Pachysolen, and Pichia [7]. In addition, it has been reported that xylose and arabinose can be fermented by genetically modified microorganisms such as S. cerevisiae, which in addition to expressing enzymes for the degradation of xylose has some advantages, such as tolerance to bioethanol and other inhibitory compounds present in lignocellulosic hydrolysates [3, 8]. These inhibitory compounds can be: acetic acid, formic acid, levulinic acid, 5-hydroxymethyl furfural and furfural, the effects caused by these inhibitors depend on the biomass used and the conditions tested during the pre-treatment [9].Simi, (2009) mentions that microorganisms grow efficiently in complex media, due to the presence of components that can be used as precursors in metabolic pathways. In the fermentative stage for the production of bioethanol, different types of nutrients can be used, many of agricultural origin such as molasses, bagasse of cane among others, which contain 45-55% of fermentable sugars like sucrose, Glucose, fructose, galactose and xylose [10]. The latter can be fermented at concentrations below 100 g/L; higher concentrations may lead to a decrease in productivity and performance of the microorganism. The effect is different for each type of microorganism being studied [11]. However, these lignocellulosic substrates may be deficient in some essential elements for growth, one of these elements is the nitrogen which is at low concentrations, making necessary the addition of a nitrogen source [12]. Similarly, sources of magnesium and phosphorus can be supplied in the form of salts along with vitamins such as biotin, thiamine and pantothenic acid, which must be supplied to the medium to increase the growth speed [12]. In the present work the capacity of three yeasts isolated from Opuntia sp. as fermenting microorganisms are evaluated, for the production of ethanol in both, defined medium and hydrolysates of Opuntia ficus indica var. Atlixco.
[3]. Since the conversion of cellulose and hemicellulose into sugar monomers such as 5 and 6 carbon carbohydrates is complicated, one of the main aspects to consider in the use of lignocellulosic residues for the production of bioethanol is the pre-treatment to destroy the cellulose matrix and dispose of fermentable sugars [4].Starting from the pretreatment or treatment of the lignocellulosic material, sugars such as hexoses are obtained, which can be efficiently converted to bioethanol with yields between 0.4 and 0.5 g/g with productivities greater than 1 g/L h when using Saccharomyces cerevisiae. Similar productivities can be obtained from mesophilic microorganisms such as Escherichia coli, Klebsiella oxytoca y Zymomonas mobilis Clostridium sporogenes, C. indolicus, C. sphnoides, Erwinia amilovora, Spirocheta aurantia, Streptococus lactis, Spirocheta litorales and Spirocheta stenostrepta [5]. However, S. cerevisiae is unable to ferment pentoses derived from hemicellulose such as xylose and arabinose [6]. The xylose is the most predominant pentose in hemicellulose, in addition, arabinose can constitute from 2-20% of the sugars in the hemicellulose depending on the agricultural residue in question [3]. Therefore, due to the high number of pentoses present in the lignocellulosic biomass, it is important to make efficient the use of these sugars by microorganisms to reduce production costs [6].Among the microorganisms that are able to naturally degrade pentoses such as xylose, are some yeasts such as Candida, Kluiveromyces, Pachysolen, and Pichia [7]. In addition, it has been reported that xylose and arabinose can be fermented by genetically modified microorganisms such as S. cerevisiae, which in addition to expressing enzymes for the degradation of xylose has some advantages, such as tolerance to bioethanol and other inhibitory compounds present in lignocellulosic hydrolysates [3, 8]. These inhibitory compounds can be: acetic acid, formic acid, levulinic acid, 5-hydroxymethyl furfural and furfural, the effects caused by these inhibitors depend on the biomass used and the conditions tested during the pre-treatment [9].Simi, (2009) mentions that microorganisms grow efficiently in complex media, due to the presence of components that can be used as precursors in metabolic pathways. In the fermentative stage for the production of bioethanol, different types of nutrients can be used, many of agricultural origin such as molasses, bagasse of cane among others, which contain 45-55% of fermentable sugars like sucrose, Glucose, fructose, galactose and xylose [10]. The latter can be fermented at concentrations below 100 g/L; higher concentrations may lead to a decrease in productivity and performance of the microorganism. The effect is different for each type of microorganism being studied [11]. However, these lignocellulosic substrates may be deficient in some essential elements for growth, one of these elements is the nitrogen which is at low concentrations, making necessary the addition of a nitrogen source [12]. Similarly, sources of magnesium and phosphorus can be supplied in the form of salts along with vitamins such as biotin, thiamine and pantothenic acid, which must be supplied to the medium to increase the growth speed [12]. In the present work the capacity of three yeasts isolated from Opuntia sp. as fermenting microorganisms are evaluated, for the production of ethanol in both, defined medium and hydrolysates of Opuntia ficus indica var. Atlixco.2. Materials and Methods
2.1. Raw Material
- Twelve-month-old Cladodes of Opuntia ficus indica, var Atlixco, were cut in 2 cm cubes of cladodes were dried at 60°C for 72 h, then milled using a commercial grain mill. The obtained flour was stored in plastic bags and kept in a dry place and mill until its use.
2.2. Acid Hydrolysis of Opuntia
- From the Opuntia ficus indica flour, acid pretreatment was performed in 500 mL Erlenmeyer flasks with a 400 mL working volume, adjusting the solids amount to 10% w/v and a 3% sulfuric acid concentration (v/v). The experimental units were maintained at 121°C and 1 atm pressure for 40 min. The obtained hydrolysates was neutralized with NaOH and centrifuged at 8000 rpm for 10 min. Subsequently, the filtrate was treated with a 0.45 μm membrane for ethanol production trials.
2.3. Microorganism, Media and Culture Conditions
- Three strains of yeasts were previously isolated from cladodes of Opuntia spp. in the laboratory of agrobiotechnology, Polytechnic University of Pachuca, Mexico. The three strains identified as Candida intermedia, Zygosaccharomyces bailii, Saccharomyces paradoxus [13] and were grown in YPDA solid medium, formulated with yeast extract (10 g/L), peptone (20 g/L), glucose (20 g/L) agar (40 g/L) after sterilization at 120°C for 15 min (Lee et al., 2000) and incubated at 20°C. The inoculum of each yeast was prepared in liquid YPD medium supplemented with an antibiotic at 75 μg/mL, incubated for 24 h at 28°C. The culture of each yeast was centrifuged at 6000 x g for 5 min to separate the biomass then it was re-suspended in isotonic solution for use.
2.4. Fermentation Kinetics in Synthetic Medium
- Yeast fermentations were performed in triplicate with a mixture of glucose, galactose, mannose, and fructose at a concentration of 20 g/L (5 g/L of each sugar) in Erlenmeyer flasks (125 mL) containing 100 mL of the fermentation medium composed of: 5 g (NH4)2HPO4, 0.75 g KH2PO4, 0.5 g K2HPO4, 0.2 g MgSO4.7H2O, 0.02 g CaCl2 2H2O, 0.1 g NaCl (Kuloyo et al., 2014), the flasks were inoculated with each microorganism by adjusting to an absorbance of 0.25 and a mixture of each of them in a 1:1 ratio. Each experimental unit was incubated on an orbital shaker at 150 rpm at 28°C for 4 days, samples of 1 mL were taken and were used to determine ethanol, reducing sugars and total sugars to each of them [14].
2.5. Fermentation Kinetics in Opuntia ficus indica Hydrolyzate
- The fermentations were carried out in Erlenmeyer flasks (125 mL) containing 100 mL of the fermentation medium obtained by acid hydrolysis supplemented with (NH4)2SO4; K2HPO4 and MgSO4. The flasks were inoculated with each microorganism by adjusting to an absorbance of 0.25, then incubated on an orbital shaker for 4 days sampling at regular intervals of time. The obtained samples were centrifuged at 13000 rpm for 5 min, the supernatant was collected and analysed for ethanol and residual sugar concentration, the residue was used for the determination of the biomass [15].
2.6. Determination of Reducing Sugars
- The concentration of reducing sugars was determined using the dinitrosalicyclic acid method described by Miller et al. (1959) and using glucose as standard.
2.7. Determination of Phenolic Compounds
- The determination of total phenolics was carried out using the Folin-Ciocalteau method described by [17] using gallic acid as standard.
2.8. Quantification of Biomass
- Biomass was determined by spectrophotometry, after preparation of the calibration curve by measuring the absorbance at 600 nm [18].
2.9. Determination of Sugars
- The concentration of glucose, galactose, mannose, and fructose and oligosaccharides in the hydrolyzate was determined by HPLC with 5 µL automatic injection per sample, using a Rezex RCM Ca+ monosaccharide column for the initial analysis and the Rezex RPM Pb+ column for the Quantification and Rezex Oligosaccharides (Phenomenex), equipped with a temperature controlled oven at 80°C, the mobile phase was water HPLC grade at 0.6 mL/min and 0.3 mL/min respectively, quantification was performed prior elaboration of calibration curves for each sugar.
2.10. Determination of Ethanol
- Ethanol determination was performed with a Trace 1310 gas chromatograph (Thermo Scientific) equipped with a Phenomenex ZB column (Phenomenex, Torrance, CA, USA) and a flame ionization detector using Helium carrier gas at 1.5 mL/min. The furnace was maintained at 40°C for 1 min, followed by a ramp at 25°C/min to 250°C with an isothermal period of 1 min, each injection was 1 μL at a Split ratio of 66: 1. The temperature of the injector was 250°C. All the supernatants were filtered through a 0.2 mm membrane prior to chromatographic analysis.
3. Results and Discussion
3.1. Obtaining Hydrolysates
- The hydrolysis process in cladode flour of Opuntia sp. Var Atlixco, with a solids loading of 10% w/v and sulfuric acid concentration of 3% v/v, with concentrations of both total reducing sugars (TRS) and phenolic compounds released (PHCR), At concentrations of 22.96 g/L and 1.63 g/L respectively, results equivalent to 27.7% of pretreatment yield, this low yield and concentration of TRS could be due, first to the degradation of sugars caused by the hydrolysis of oligosaccharides to compounds of low molecular weight and the subsequent degradation of the products obtained to furfural derivatives [19, 20] and second, to the solids loading in the acid pretreatment [21, 22]. In addition, the effect of a second and third hydrolysis was determined under the same conditions, with a decrease in the amount of TRS at 6 and 2 g/L for each subsequent hydrolysis stage, respectively, with an increase in the yield of the process up to 87.61%, Under the amount of solids used, [23] observed yields of up to 50% with 50 g / L of TRS for the lignocellulosic residue used. On the other hand, in a study conducted by Guevara-Figueroa et al. (2010) to determine the lyophilized phenolic compounds content of ten varieties of cactus, found that the phenolic amount present in the samples was from 2 mg/g to 20 mg/g. Additionally, [25] observed concentrations of phenolic compounds from 0.3 g/g to 0.9 g/g under drying conditions, conditions similar to those submitted to the Opuntia ficus indica variety studied in the present work. In addition, the chromatographic analysis of Opuntia ficus indica hydrolysates showed the presence of oligosaccharides such as xylopentosa, xylotetroses, xylotriose, maltose and xylobiose (Table 1). Additionally, it was observed that this amount of both monosaccharides and oligosaccharides is variable depending on the pre-treatment conditions used. The presence of these oligosaccharides may be due mainly to the acid treatment, eliminating the insoluble hemicellulose from the surface of cellulose microfibrils giving soluble oligosaccharides [26].
|
3.2. Fermentation Kinetics and Sugars Assimilation in Defined Medium
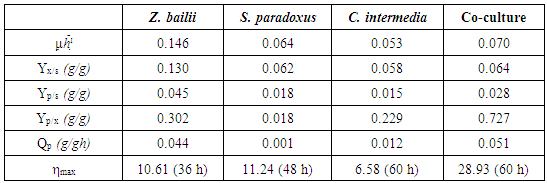
- The previously isolated strains were evaluated for their ability to ferment and assimilate carbon sources such as glucose, mannose, galactose and fructose in a synthetic medium at a concentration of 20 g / L in a mixture of sugars. From the kinetics, it was observed that the Z. bailii strain, preferably consumed fructose, followed by glucose and mannose, during the first 25 hours of growth, in the same way, it was observed that there was a limitation in the consumption of galactose (Figure 1A). On the other hand, the strain S. paradoxus presented limitation to the mannose consumption, observing a preferential consumption of glucose, followed by galactose and fructose (Figure 1B), while C. intermedia showed a gradual consumption of sugars, being mainly glucose and fructose the most consumed sugars followed by mannose and galactose. (Figure 1C). When evaluating the effect of isolated strains inoculated as a consortium (Fig. 1D), it was observed that the consumption of sugars as the only source of carbon was complete after 50 h of culture. The consumption of sugar was mainly of glucose, during the first 5 h, followed by fructose. These results suggest that Z. bailii and C. intermedia strains were the main microorganisms that metabolized these sugars, finally, the consumption of mannose and galactose was observed without having detected a significant increase in the production of ethanol, so it is very likely that these last two sugars are used as maintenance of the microorganism. Whereas the S. paradoxus strain had little assimilation of sugars, which indicated that this yeast requires special elements in the medium as growth factors, vitamins or culture conditions that allow both, the activation of the necessary enzymes as their assimilation and the obtaining of primary and secondary metabolites. [27] observed that under conditions of aerobic growth, both Kluyveromyces marxianus and S. cerevisiae preferentially consume glucose and fructose and when the conditions of the medium are changed to anaerobic, the microorganism is able to metabolize galactose, xylose, and arabinose, in addition, they observed that this consumption increases at the end of easy-to-assimilate carbon sources.
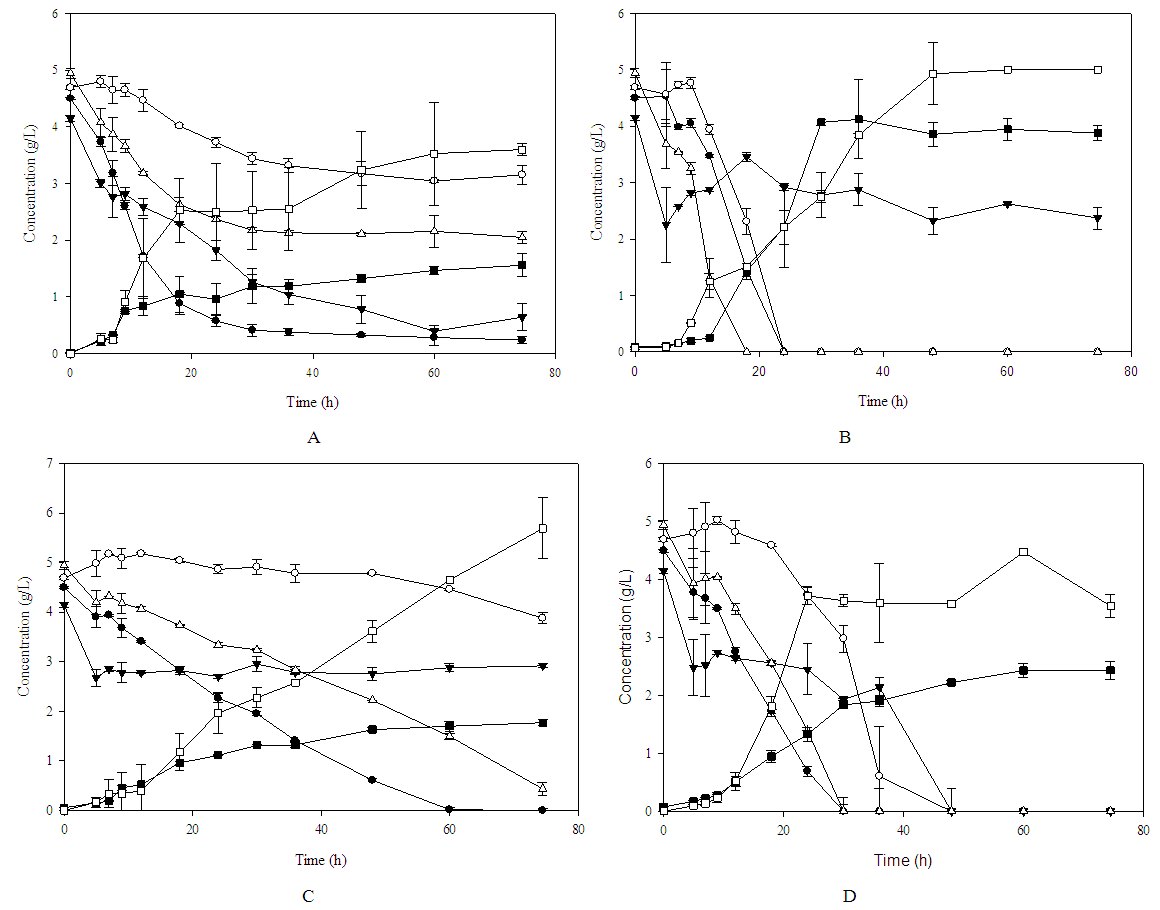 | Figure 1. Sugar consumption kinetics for yeast strains;  glucose; glucose;  manose; manose;  galactose; galactose;  fructose; fructose;  Biomass; Biomass;  ethanol. (A) Z. bailii; (B) S. paradoxus; (C) C. intermedia; (D) co-culture ethanol. (A) Z. bailii; (B) S. paradoxus; (C) C. intermedia; (D) co-culture |
|
3.3. Fermentation Kinetics in Opuntia ficus indica Hydrolyzate
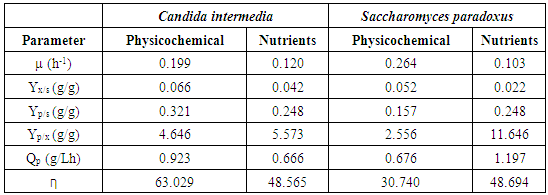
- Previous studies (data not shown) allowed to adjust several environmental conditions as well as the addition of nutrients for each of the yeasts studied. Conditions such as pH of 6.5, 150 rpm of stirring and 30°C were adjusted as conditions, allowing the maximum ethanol production for C. intermedia yeast, while for the addition of nutrients the KH2PO4 salt was evaluated as the only additional nutrient at a concentration of 4 g/L. Figure 2B shows the kinetics performed during 72 h, where it was observed that the maximum amount of ethanol that could be obtained was 3.5 g/L and 4 g/L for the addition kinetics of P (phosphorus) as an additional nutrient and environmental conditions respectively (Figure 2A). These amounts were slightly compared to that obtained using a defined medium, this effect could be due to the process of adaptation of yeast cells to the medium used and the presence of various compounds in the medium such as phenolic compounds, which were 1.63 g/L in the hydrolysates, since the microorganism was grown initially in nutrient-rich medium (YPD).
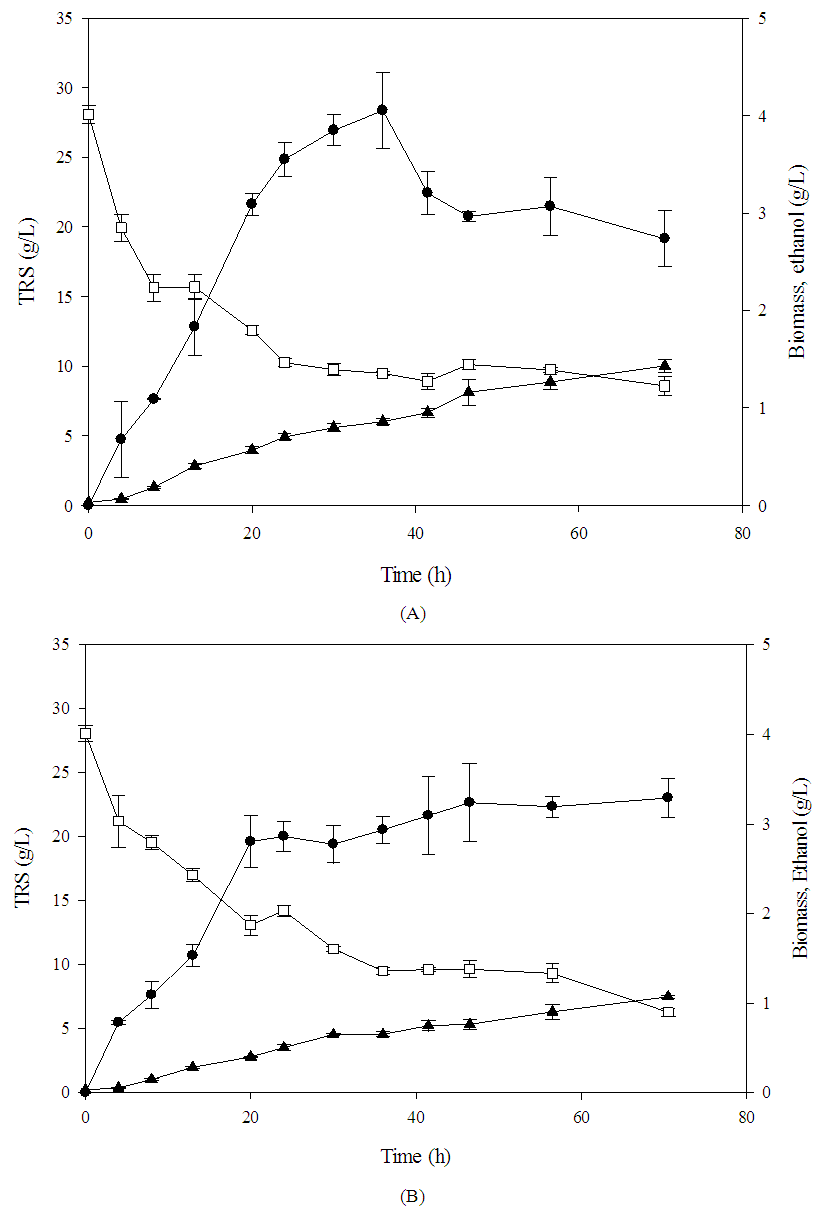 | Figure 2. Ethanol production kinetics for C. intermedia with physicochemical (A) and nutritional (B) factors.  Biomass, Biomass,  Ethanol, Ethanol,  TRS (g/L) TRS (g/L) |
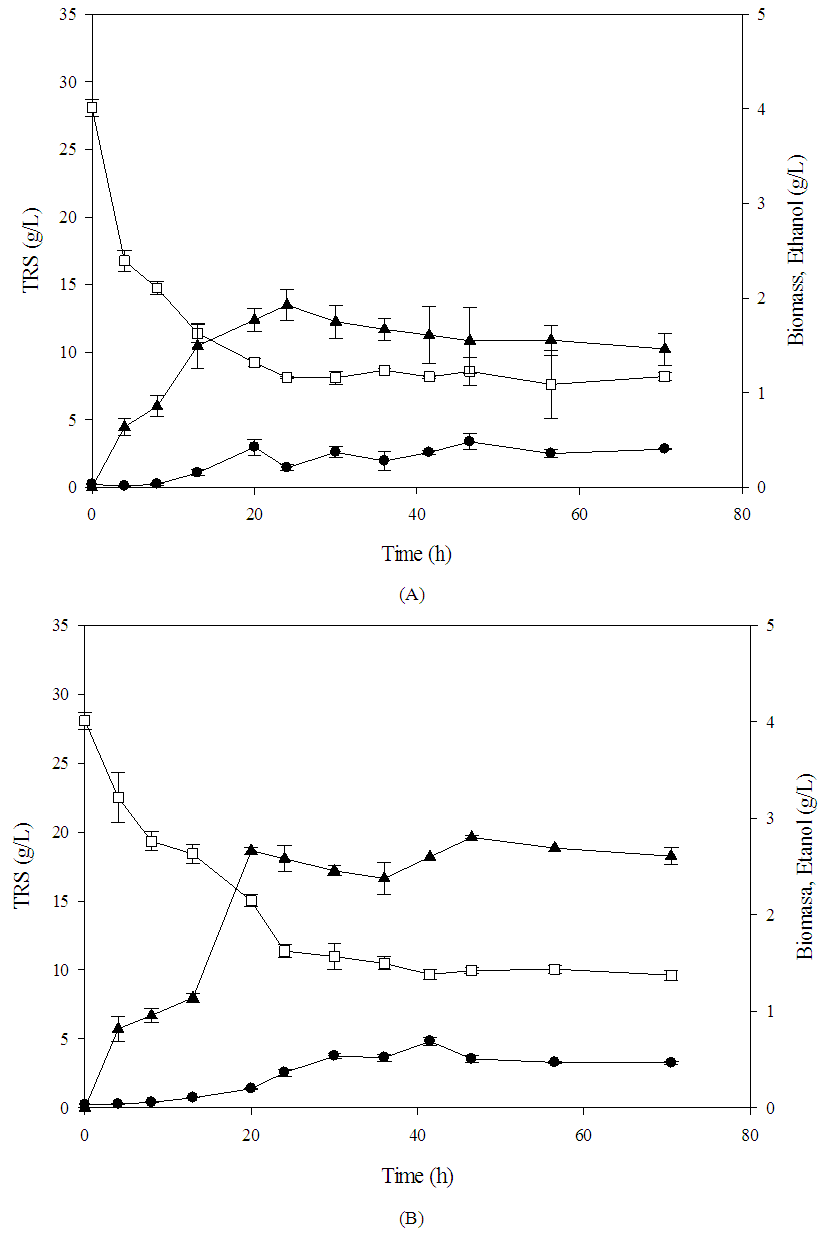 | Figure 3. Kinetics of ethanol production for S. paradoxus, in physicochemical (A) and nutrients (B) factors.  Biomasa, Biomasa,  Ethanol, Ethanol,  TRS (g/L) TRS (g/L) |
|
4. Conclusions
- Based on the results obtained, it was possible to determine that the yeast C. intermedia is a good candidate to be used in the production of ethanol under different culture conditions, since ethanol production was observed in both synthetic medium and in hydrolysates obtained from Opuntia ficus indica as a culture medium, however, it is necessary to increase the release of fermentable sugars to improve yields, so as to allow the use of Opuntia ficus indica as a possible candidate suitable for the production of biofuels. During the hydrolysis of Opuntia flour, the loading of solids is essential to obtain a greater quantity of free sugars. The more solids, the hydrolysis time should be longer but with the possibility of releasing more toxic compounds (such as furfurals). Therefore, it is necessary to complement the chemical hydrolysis with a biological hydrolysis or complement with other physicochemical methods to increase the release of sugars. It was observed in the hydrolysates, the presence of a great variety of oligosaccharides product of the hydrolysis of the hemicellulose and mucilage that can be an indicative of a partial hydrolysis. A limitation of the process is the chemical complexity of the hydrolysates which avoids high ethanol yields, hence the need to use microorganisms that are capable of fermenting most of the carbohydrates present in the hydrolyzate that would be possible with the use of microorganisms genetically modified.
ACKNOWLEDGMENTS
- This work was conducted thanks to the support received from Mexico’s National Science and Technology Council (CONACyT) and Sectorial Fund SAGARPA-CONACyT, project No. 195157.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML