-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2018; 8(1): 4-9
doi:10.5923/j.ijaf.20180801.02

Environmentally Sustainable Management of Water Hyacinth (Eichhornia crassipes) in Guyana
Jonelle Cornette1, Clairmont Clementson2, David Fredericks2
1Research Assistant, University of Guyana, Turkeyen, East Coast Demerara, Guyana
2Research Scientist, National Agricultural Research and Extension Institute, Mon Repos, East Coast Demerara, Guyana
Correspondence to: Clairmont Clementson, Research Scientist, National Agricultural Research and Extension Institute, Mon Repos, East Coast Demerara, Guyana.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The increase of greenhouse gasses and chemical fertilizer use due to the increasing energy needs and industrial revolution have contributed to climate change concerns prompting the need for alternative environmentally friendly and renewable sources. Water Hyacinth is a rapid growing water weed whose management presents a challenge to the drainage system and a breeding area for mosquitoes thereby enabling the spread of diseases. This research seeks to highlight an opportunity to derive economic and environmental benefits from water hyacinth thereby mitigating against environmental management issues. It characterizes biochar produced from water hyacinth at three pyrolysis temperatures and examined its suitability as a soil amendment along with its energy and carbon capture potential. Increasing the pyrolysis temperature transformed the biomass matrices into a lighter and porous structure causing a decrease in density. There was an increase in ash and fixed carbon content while volatile matter decreased as pyrolysis temperature increased indicating a higher concentration of organic matter are available for carbon sequestration at higher pyrolytic temperatures. Further, the presents of micro-nutrients and high pH makes biochar from these water hyacinth samples suitable as a liming agent and a soil amendment for vegetables, legumes and grains in open-field or shaded conditions. Additionally, the biochar is recommended as a fertilization and soil improvement additive to aid Guyana’s agricultural production expansion into its Hilly Sand and Clay Region.
Keywords: Water hyacinth, Biochar, Soil amendment, Carbon sequestration, Drainage control
Cite this paper: Jonelle Cornette, Clairmont Clementson, David Fredericks, Environmentally Sustainable Management of Water Hyacinth (Eichhornia crassipes) in Guyana, International Journal of Agriculture and Forestry, Vol. 8 No. 1, 2018, pp. 4-9. doi: 10.5923/j.ijaf.20180801.02.
1. Introduction
- The increase in greenhouse gas emissions from energy needs worldwide has prompted significant research into the development of renewable energy sources. These include the conversion of biomass into biofuels and other value-added renewable products. Biomass is any organic matter produced by photosynthesis existing on the earth’s surface. It is the naturally occurring energy-containing carbon resource that is large enough in quantity to be used as a substitute for fossil fuels (Balat et al., 2009). One such biomass derivative, biochar, has been proven to be a valuable resource for carbon sequestrate also a medium for the immobilization of organic contaminants in soil (Cao et al., 2009). It reduces the need for chemical fertilizer, resulting in decreased emissions from fertilizer production, increases soil microbial life which results in more carbon storage in soil while retaining more nitrogen. Biochar is a fine-grained porous substance obtained from the pyrolysis of biomass. Pyrolysis is a relatively simple technique in which organic material is heated in the absence of oxygen. During the pyrolysis process, the natural polymeric constituents (i.e. lignin, cellulose, fats and starches) are thermally broken down into three different fractions: bio-oil (condensed vapors), char (solid fraction) and non-condensable gases. The solid or char fraction obtained from biomass pyrolysis is often considered a waste product and combusted to provide the necessary heat for the pyrolysis process. However, recent research suggests that char can be used as a soil amendment, hence termed biochar, to substantially increase soil fertility (Lehmann et al., 2006). The use of biochar for remediation is not a new concept since it has been found to be an ancient practice in areas of the fertile Amazon Basin. The terra preta soils of the region received large amounts of charred organic debris from the former inhabitants. As a result, carbon stocks have remained in the soil, even thousands of years after abandonment (Kwapinski et al., 2010; Lehmann et al, 2006).In Guyana water hyacinth (Eichhornia crassipes) has impeded water flow in irrigation and drainage canals resulting in eutrophication and provide or enhance breeding places for disease-carrying mosquitoes. It exhibits rapid growth and can spread quickly making it very difficult to control once it becomes fully established. Integrated control of water hyacinth can ensure economic and environmental benefits while keeping the population of this weed in check. This study proposes the use of water hyacinth to produce biochar which can then be used as a soil amendment and carbon sequester. It’s suitability as an organic fertilizer is premised on its low carbon: nitrogen ratio (C:N) of about 1:24.3 with a lignin content of only 9% compared with C: N ratio of about 1:80 and lignin content of 17% for other biomass such as wheat straw (Mallik et al., 1990). The weed is a good absorber of nitrogen, phosphorus and potassium from water. Further Jafari (2010) posited that on a zero-moisture basis, water hyacinth soil amendment contains as high as 75.8% organic matter, 1.5% N, and 24.2% ash. Additionally, with the ash containing 28.7% K2O, 1.8% Na2O, 12.8% CaO, 21.0% Cl, and 7.0% P2O5. Hence the general objective of this study is to assess the suitability of water hyacinth (Eichornia crassipes) biochar as a soil amendment. The specific objectives are: (i) To characterize the physico-chemical properties of water hyacinth (Eichornia crassipes) prior to biochar production, (ii) To characterize the physico-chemical properties of water hyacinth (Eichornia crassipes) after biochar production, and (iii) To determine the biomass to biochar ratio and value of biochar as a fertilizer.Biochar production by pyrolysis of biomass effectively removes carbon from the atmospheric carbon cycle, transferring it to long-term storage in soils. Biochar, therefore, could help in the global challenge of carbon dioxide (CO2) mitigation, as it results in a net removal of carbon from the atmosphere (Lehmann and Joseph, 2009), hence, the recalcitrant weed then becomes a valuable resource.
2. Material and Methods
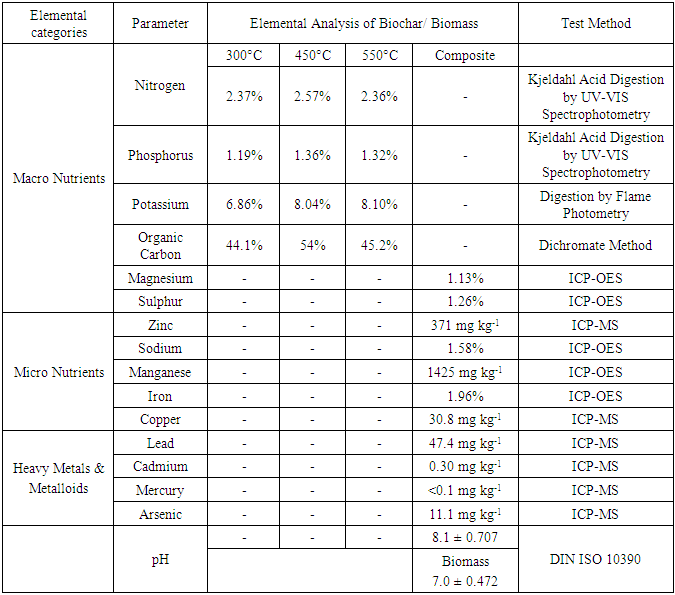
- Fresh water hyacinth was manually collected from the Dennis Street drainage canal in the Sophia area (latitude: 6.8169°; longitude: -58.1267°) of Georgetown, Guyana. This canal has an accumulation of surface runoff groundwater along with industrial and residential wastewater that provides an ideal environment for the growth of the water hyacinth plant. This canal is often clogged up with water hyacinth that prevents the free flow of water.The water hyacinth was taken to the Institute of Applied Science and Technology within the University of Guyana’s Turkeyen compound where they were cut into small pieces of approx. 20–40 mm, air dried 18 days with average weather conditions of 30° /22°C (daytime/nighttime) temperatures, 78% humidity and 35% cloud cover in daylight. The biomass was then placed in sealed (50kg) polythene bags and labelled WH300, WH450, and WH550 samples referring to the pyrolysis temperatures of 300°C, 450°C and 550°C used. These temperatures were chosen because different classes of complex organic polymers tend to decompose at varying peak temperatures based on structural composition. The biomass was ground/milled to pass through a 1 mm sieve with all respective equipment calibrated. The moisture content, ash content, volatile matter and fixed carbon of the grounded water hyacinth biomass was determined according to American Society for Testing and Materials (ASTM) testing codes E1756-01: Standard Test Method for Determination of Total Solids in Biomass, E1755-01: Standard Test Method for Ash in Biomass, E872-82: Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels and 870-82: Standard Test Method for Analysis of Wood Fuels respectively.To produce the biochar samples the crucibles were firstly sanitized, air dried then oven dried at 105°C for 1 hour. They were then removed, cooled in desiccators and weight recorded to the nearest 0.1 mg (crucible weight). 100g of ground samples in triplicate were placed in crucibles and this weight was recorded as the initial weight. The furnace was initially heated 250°C then at approximately 5°C/min to 300°C. The crucibles with raw biomass was placed in the furnace and carbonized for 40 minutes. Crucibles were removed from the furnace, cooled to room temperature in desiccators and final weight (final weight) was recorded to the nearest 0.1 mg. Biochar yield was then calculated using the formula: Biochar Yield (%) = (Final weight -weight of crucible) / (Initial weight – weight of crucible) × 100% The volume of biomass to biochar ratio was assessed by first determining their specific volume by the relationship, v = ρ-1 where the specific volume (v) of a substance is the reciprocal of density (ρ). This being an intrinsic property per unit mass.Biochar was then produced at carbonization temperatures of 450 and 550°C following the same procedure. These temperatures fall within the range of slow pyrolysis and lignin decomposition.After carbonization, the biochar was tested according to standards recommended by the International Biochar Institute (IBI) Biochar Standard version 2.0. The moisture content, ash content and volatile matter analysis of the biochar were conducted in triplicate using the ASTM D1762-84: Standard Test Method for Chemical Analysis of Wood Charcoal. The fixed carbon content and bulk density analysis were done according to ASTM E870-82: Standard Test Method for Analysis of Wood Fuels and ASTM D7481-09: Standard Test Methods for Determining Loose and Tapped Bulk Densities of Powders using a Graduated Cylinder respectively.Data collected were tabulated and statistical analyses were done to establish whether there was correlation between the physicochemical properties of the biomass and biochar, and further the trends of the biochar properties for different pyrolysis temperatures.Organic Carbon analyses were performed using Dichromate Method at the Guyana Sugar Corporation Inc. (GuySuCo) laboratory (La Bonne Intention, East Coast Demerara, Guyana) while macro and micro nutrients were determined using Kjeldahl Acid Digestion, Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) along with Inductively Coupled Plasma Mass Spectroscopy (ICP-MS) methods and pH was conducted using the DIN ISO 10390:2005 method at Activation Laboratories Limited (41 Bittern Street, Ancaster, Ontario L9G 4V5).
3. Results and Discussion
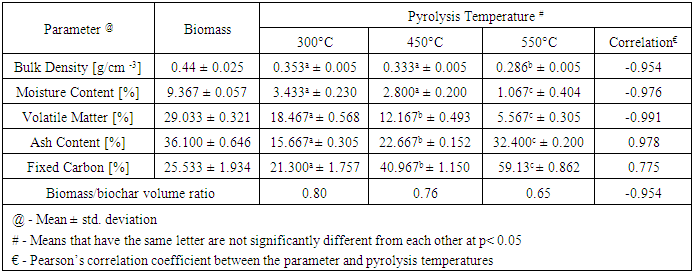
- Biochar yield negatively correlated (R2 = 0.93) with increasing pyrolysis temperature (Figure 1). The highest yield of biochar (62.2%) was obtained at 300°C and decreased to (35.9%) as the temperature increased to 550°C. From this data, biochar yield (y) from water hyacinth by pyrolysis could be predicted by the regressive equation for pyrolysis temperature (x) as y = -0.1096x + 93.484.
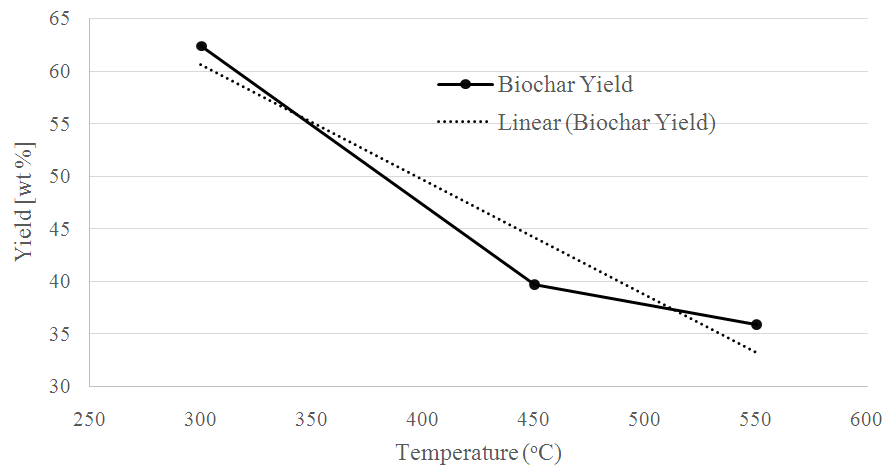 | Figure 1. The effect of pyrolysis temperature on biochar yield, expressed in wt. % of biomass feedstock |
|
|
4. Conclusions
- This research seeks to highlight an opportunity to derive economic and environmental benefits from water hyacinth thereby mitigating against environmental management issues. In this study biochars were produced from water hyacinth at three pyrolysis temperatures (300, 450 and 550°C), and their properties characterized. Laboratory experiments were conducted to investigate how the physicochemical properties of the biomass change due to thermal processing. Increasing the pyrolysis temperature transformed the biomass matrices into a lighter and porous structure causing a decrease in density. There significant difference in the ash content, volatile matter and fixed carbon obtained from the pyrolysis temperatures (p≤0.05). Ash content and fixed carbon content increased while volatile matter decreased with pyrolysis temperature indicating a higher concentration of organic matter available for carbon sequestration at higher pyrolytic temperatures. Further, the presents of micro-nutrients and high pH makes biochar from these water hyacinth samples suitable as a liming agent and a soil amendment for vegetables, legumes and grains in open-field or shaded conditions. Also, the biochar is recommended as a fertilization and soil improvement additive to aid the expansion of Guyana’s agricultural production into its Hilly Sand and Clay Region. Field trials should be done to compare the efficacy of this soil amendment to chemical fertilizer and other natural sources.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML