Yaema Fornah1, Stephen Brima Mattia2, Adegboyega Ayodeji Otesile2, Ernest G. Kamara3
1Sierra Leone Agricultural Research Institute, Kenema Tree Crops Research Centre, Sierra Leone
2Department of Forestry, School of Natural Resources Management, Njala University, Sierra Leone
3Sierra Leone Agricultural Research Institute, Njala Agricultural Research Centre, Freetown, Sierra Leone
Correspondence to: Ernest G. Kamara, Sierra Leone Agricultural Research Institute, Njala Agricultural Research Centre, Freetown, Sierra Leone.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Abstract
An investigation was undertaken at Njala, in Sierra Leone to assess the effect of provenance and seed size on germination and physiological traits of Gmelina arborea. The objective of the study is to identify seed size and sources that are superior in terms of important characteristics that would be the best sources for seed improvement programmes and production of Gmelina trees. Seeds of Gmelina were collected in 2014 from four geographically distinct provenances in Sierra Leone. The experiment was 4 x 3 factorial laid in a randomized complete block design with four replications four levels of provenance (seed origins), North, South, East and West and three levels of seed sizes – Small, Medium, and Large. The results showed that both germination percentage and germination rate of Gmelina were significantly affected by seed size. The large and medium seed sizes from different provenances had significantly higher germination percent and germination rate than the small seed size. Highly significant (P < 0.001) differences were observed in germination percentages among the different seed sizes at 2 and 3 weeks after planting (WAP). The large seed size which had the highest germination percentage at 2 WAP (66.4%) and 3 WAP (78.1%) followed by the medium size seeds at both 2 WAP and 3 WAP (61.4% and 74% respectively). Results of the correlation analysis showed a strong positive correlation (r = 0.91) between seedling height and total biomass. Similarly, a positive correlation was observed between shoot dry weight and total biomass (r = 0.72) while as the correlation of root dry weight and total biomass was very weak (r = 0.45). Provenance significantly affected shoot dry weight and total biomass of Gmelina seedlings. Seedlings from seeds obtained from the East and Southern regions consistently had higher shoot dry weight and total biomass than seeds obtained from the North and Western regions.It can be concluded that seed size is a major parameter for predicting germinabilty and seedling establishment of Gmelina. The best seed sizes to use by tree planters and other stakeholders were the large sized seeds because of their fast germination and seeds from the east and south will promote early maturity of Gmelina arborea for purposes such as pulp paper production, timber and fuel wood.
Keywords:
Seed size, Provenance, Germination, Seedling morphology, Growth rate
Cite this paper: Yaema Fornah, Stephen Brima Mattia, Adegboyega Ayodeji Otesile, Ernest G. Kamara, Effects of Provenance and Seed Size on Germination, Seedling Growth and Physiological Traits of Gmelina arborea, Roxb, International Journal of Agriculture and Forestry, Vol. 7 No. 1, 2017, pp. 28-34. doi: 10.5923/j.ijaf.20170701.05.
1. Introduction
Growing seedling in the forest nursery is the main way of raising planting stocks in the tropics for plantation establishment. Few plantation species are extensively propagated vegetatively. Therefore, obtaining adequate amounts of the right kind of seed is an important part of any plantation programme [1] (Evans, 1986).The increase in the cost of imported wood products explains the need for intensification of the establishment of some exotic species into plantations in favour of slow growing indigenous species to produce raw materials for wood based industries in tropical countries. Presently, national forests are being depleted of their goods and services, which would have been derivable from them because of anthropogenic factors. Even though seed is a renewable natural resource, once the source it is exhausted, it may take a long time to regenerate. Ogigirigi (1989) [2] found out that the original vegetation, especially in savannah zones has been modified by human activities over the years; this has made the vegetation lose its original form. To correct this situation, plantations of economic indigenous and exotic species must be established to regain the vegetation and prevent desert- like conditions.There is still need for a systematic and up-to-date account of the principles of forest trees handling and their application to the special problems of tropical forest seeds. A successful plantation cannot be established unless healthy nursery seedlings or stocks are produced. This also may depend on the viabilities and seed sizes [3] (Kadambi, 1972). The need for seed has considerably increased in the tropics with the unprecedented expansion of afforestation and reforestation programmes. Seed size is parameter for predicting germination and seedling growth rates, both in the nursery and for a brief period following plantation establishment [4] (Oni and Bada, 1992). Seed also forms a very important element in the quality of seedlings produced in the nursery, since the quality of the seedlings is determined by the genotype of the seed from which it originates. Therefore, the effect of seed sizes and provenance in early morphological and physiological characteristics of Gmelina arborea has become necessary to be considered and determined [5, 6] (Adegbehin et al., 1988; Owoh et al., 2011).Provenance studies in forest trees are very important as it helps in identifying the best and highly adaptable provenance. In fact forest tree improvement programme starts with the scanning of provenances/ seed source capable of providing best-adapted trees [7] (Suri, 1984). Increase in yield and resistance to disease can be achieved through the selection and use of seed from good provenance. Seed source studies are also desirable to screen the naturally available genetic variation to utilize the best material for maximum productivity and for further breeding programme [8] (Shiv Kumar and Banerjee, 1986).Although many tree planters considered Gmelina to be a very promising species due to ease and low cost of establishment, rapid early growth, expectations of early returns and promising wood characteristics, including high durability and good yield and quality of pulp, some tree planters have had a less encouraging experience with the species [9] (Lauridsen and Kjaer, 2002). They have found a rapid reduction in increment after the seventh year of growth [9] (Lauriden and Kjaer, 2002). Gmelina species has generated interest because of its fast growth and quick return on investment. Its wood can be used for a multitude of products that range from pulp to furniture parts. It also has great utility in agroforestry systems [10] (Dvorak, 2003). A successful plantation cannot be established unless healthy nursery seedlings or stocks are produced. There is growing increase in the rate of deforestation in country as a result of farming, charcoal burning, sawing of timber and mining activities. Although there is increase deforestation and cutting down of trees, very little effort has been made to replenish trees especially those in reserve forests.The increased rates of tree planting, which is apparent in so many countries today, emphasizes more than ever before the need for good seed. Seed quality has a critical effect on the quality of the trees established and on the economics of planting them. This is equally true whether planting is in large-scale commercial plantations or in small-scale diffuse farm woodlots or as scattered single trees. Seed quality comprises both the physiological viability and vigor of the seeds and their genetic quality – their ability to produce healthy offspring which are well suited both to the site where they are planted and for the products or services which they are intended to provide [11] (FAO, 1987).To investigate the effects of seed size and sources on the growth performance of Gmelina arborea; with a view to identifying best sources of seeds of this species for future production needs or for seed improvement in Sierra Leone. Specific objectivesi. To determine the effect of seed size on germination of Gmelina arborea.ii. To determine the effect of seed size on seedling development of Gmelina arborea.iii. To assess the variations in early seedling growth among seedlings obtained from the different provenances.
2. Materials and Methods
2.1. Description of Study Area
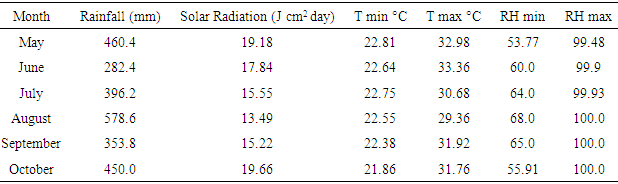
The study was carried out at Njala University, Njala campus which is located in Kori chiefdom in Moyamba district. The district is geographically located along the South-Northern axis of the country. This area has an estimated population of 29,043 (Statistics Sierra Leone, 2004). Njala is located at an elevation of 54m above sea level on 8°06' N latitude and 12°06' W longitude.The predominant vegetation at Njala is secondary forest. The upland soils in the Njala area have been classified as Orthoxic palehumult, belonging to the Njala series [12] (Odell et al., 1974). Textures are usually gravely clay loam in the surface and gravely clay in the subsoil. Table 1. Weather data of study area – 2014
 |
| |
|
Njala experiences a distinct dry and wet season. The monomodal rainy season last from April to November, while the dry season extends from December to March. The mean monthly air temperature ranges from 21°C - 23°C maximum for the greater part of the day and night. Average annual precipitation of the district ranges from 3,330mm to 4000mm with mean maximum temperature of 29°C to 30°C [13] (Jusu, 1990).
2.2. Seed Collection and Processing
Seeds of Gmelina were collected in 2014 from four geographically distinct provenances in Sierra Leone. Seed collection was done in Freetown; (coastal plain, in the Western Area Peninsula Forest Reserve “WAPFR”), Makeni; (Kangari Hill Forest Reserve), Moyamba; (Kasiwi Forest Reserve) and Kenema; (Kambui Hill Forest Reserve). All the four agro climatic zones were sampled for Gmelina seeds. Mature fruits of Gmelina arborea, were collected from the mother trees in Freetown, Makeni, Moyamba and Kenema towns. The seeds were depulped, washed and sun-dried for 24 hours to enhance germination.
2.3. Experimental Design and Cultural Practices
The experiment was established at the Department of Forestry nursery site at the Njala campus of Njala University, in May 2014. The experiment was a 4 x 3 factorial; laid in a randomized complete block design with four replications representing the four provenances (North, South, East and West and three levels of seed sizes – Small, Medium, and Large. These resulted in 12 Treatments. A total of 100 seed samples were collected from each agro climatic zone (provenance) for the study The seeds were randomly selected and grouped into three size classes namely, large seed size (LSS), medium seed size (MSS) and small seed size (SSS), based on the seed fresh weight). Seeds of 0.94g were considered large while seeds of 0.57g and 0.38g were considered medium and small respectively [14] (Offiong, 2008).The selected seeds were sown in black polythene bags filled with sterilized river soil. Watering was done twice daily. Germination count was done daily for three weeks. Germination percentage of each treatment was calculated by dividing the number of germinated seedlings against the number of seeds sown and multiplied by one hundred.Seedling height was measured with a metre rule from soil level to the topmost leaf axil of the main stem at intervals of 2, 4, 6, 8 and 10 weeks after planting and their mean height was expressed in centimeter. Leaf area was determined by measuring the leaf area of three leaves using the graphical method (graph paper). Dry weight - dry weight was measured from 3 plants randomly harvested for each treatment by destructive sampling at 1, 2 and 3 months after planting, plants were dried in an oven for 2 days at 80°C for 24hrs and the dry weight were measured using an electronic weighing balance. Mean dry weight per plant was recorded per grams.
2.4. Data Analysis
Data collected on germination and early growth parameters such as seedling height, leaf number, leaf area and stem diameter (collar) were subjected to analysis of variance (ANOVA) using Genstat Release version 7.2DE. Means were compared with Least Significant Difference (LSD) at 5% probability Duncan’s Multiple Range Test (DMRT) was used for pair comparisons. The residuals of data for the parameters used were first checked for normality and homogeneity using the Shapiro-Wilk test and the Bartlett’s test respectively for both experiments. Data collected on germination count were transformed using square root transformation. Germination rate was calculated using the formula. N2 – N1/t1 –t2 where N1 = initial germination count and N2 = final germination count.t1 = initial time (in day) t2 = final time (in days). Data on dry weight at 1, 2 and 3 MAP were used to calculate Relative Growth Rate and Average Growth Rate using the formulae below (Offiong, 2008) Relative Growth Rate (RGR)- RGR = In W2-In W1/T2-T1 Where W1 = represented the dryness of previous sampling and W2 the dryness of the sample that followed. T1 and T2 above the corresponding initial time and final time (in months) of sampling respectively In = natural log. Average Growth Rate (g/month) = TDM2 – TDM1/ t2-t1 where TDM1 = Initial total dry weight TDM2 = Final total dry weightt1 = initial time (in months)t2 = Final time (in months)Pearson's correlation analysis was explored to examine relationships among the following parameters - Germination percentage, leaf number, leaf area, seedling height, stem diameter, root and shoot dry weights and total biomass.
3. Results and Discussion
3.1. Germination Assessment
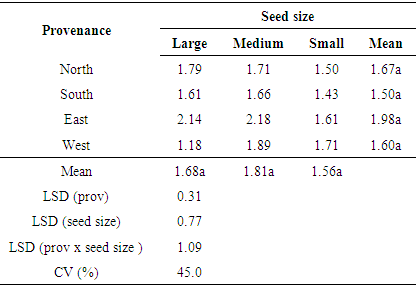
Highly significant (P < 0.001) differences were observed in germination percentages among the different seed sizes at 2 and 3 weeks after planting (WAP) The large seed size had the highest germination percentage at 2 WAP (66.4%) and 3 WAP (78.1%) followed by the medium size seeds at both 2 WAP and 3 WAP (61.4% and 74 % respectively). The small size seed had the least germination percentages at both 2 WAP (54.4%) and 3 WAP (63.4%), There was no interaction effect (P = 0.6 and 0.66 respectively) between seed size and provenance at 2 and 3 WAP (Week after planting). The results showed that both germination percentage and germination rate of Gmelina were significantly affected by seed size. The large and medium seed sizes from different provenances had significantly higher germination percent and germination rate than the small seed size. These results are in conformity with earlier results obtained by Oni and Bada [4] (1992) who also reported seed size is a parameter for predicting germination and seedling growth. The superior germination exhibited by the large and medium size seed over the small size seeds could be attributed to availability of more food reserves in large seeds. This enhanced their viability, hence higher germination percentage and germination rate. Similar observations were made by [14] Offiong (2008) who reported that seeds with large dimensions are likely to have large embryo which enhances good germinability of Gmelina seeds. [15] Khan et al. (2009) also reported that during the establishment stage, the embryo grows at the expense of food materials and did not require any external nutrition. On the other hand, the lower germination obtained from small size seeds could be due to the relatively lower food reserves in the small seed size due to their small size of their cotyledons and origin of seeds. [16] Zaidman et al, (2010) suggested that improved seed and seedling quality, as associated with greater seed weight can be attributed to better membrane integrity and increased availability of energy.Generally, the results showed that there was no significant (P < 0.05) difference in germination rates among the different seed sizes and seed sources (provenance) of Gmelina (Table 2). The medium size seeds had the highest rate of germination (1.81 per day) followed by large seeds (1.68 per day) whilst the small seeds had the least germination rate. The results also showed that the highest germination rate (1.98 per day) was obtained from seeds sourced from the eastern region of Sierra Leone. The lowest germination rate was from seeds sourced from the south. However, the correlation between germination percent at 3 WAP and germination rate from 2 to 3 WAP after planting was very weak (r = 0.59).Table 2. The effect of provenance and seed size on germination rate of Gmelina seeds
 |
| |
|
3.2. Assessment of Growth Parameters
3.2.1. Seedling Height
Seedling height differed significantly with seed size at (P< 0.05), but with the reverse was observed in relation to the provenances at different weeks after planting except at 2 WAP (P=0.84). The large size seed had higher seedling heights at 4, 6, 8 WAP (11.2, 19.8 and 31.8 cm respectively) except at 2 and 10 WAP where no significant differences were observed between medium and large seeds (Table 3). No significant difference was observed in seedling height among seeds obtained from different Provenances. The results generally showed that seed size significantly influenced seedling height at different weeks after planting. There is a general increase in early growth trait with time after planting. The results showed that seedling height increased rapidly from 2 to 4 weeks after planting. The large seed size had 146% increases from 2 to 4 weeks whilst the medium and small seed sizes increased by 106% and 104% respectively (Table 3). The fast establishment of seedlings from large seeds could give an early advantage to compete with weeds as compared to seedlings from small seeds. [17] Gonzalez (1993) stated that seed size affected plant vigor as seeds with greater mass produced more vigorous plants. Results of the present study showed that large and medium seed sizes had significantly higher values of seedling height.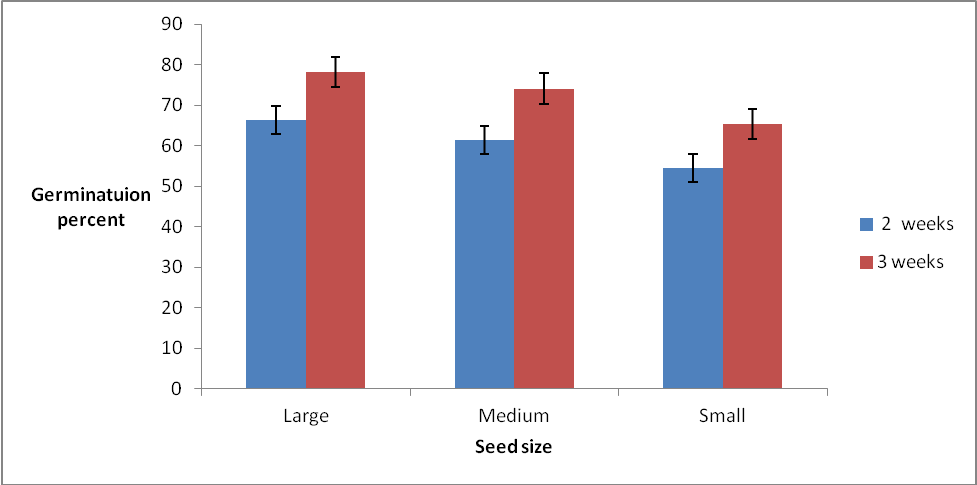 | Figure 1. Effect of seed size on germination percentage of Gmelina arborea |
Table 3. Effect of Seed Size on Seedling Height of Gmelina arborea
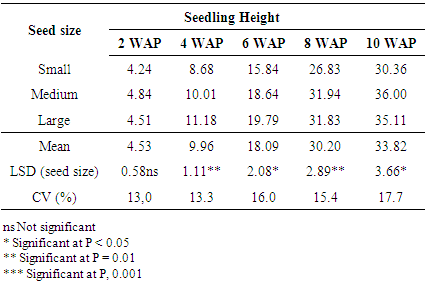 |
| |
|
3.2.2. Leaf Area of Gmelina Seedlings
Leaf area significantly (p < 0.05) differed with seed size at the different weeks after planting except at 4 WAP (p = 1.10). The leaf area increased consistently from 2 to 10 WAP (Table 4) in all the seed sizes. The large size seeds had the largest leaf area in the different WAP followed by the medium size seeds. The small seed size had the lowest leaf area in the different WAP recorded (Table 4). Results of the present study showed that large and medium seed sizes had significantly higher values of leaf area than the small size seeds. Similar results had earlier been reported by [18] Boot (1996) who found that bigger seeds produce bigger seedlings with larger area of green leaves capable of conducting photosynthesis.Table 4. Effect of seed size on leaf area of Gmelina arborea
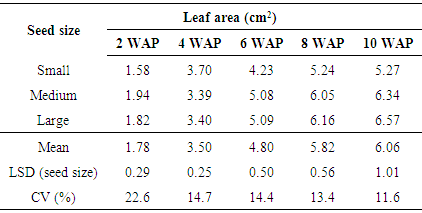 |
| |
|
3.2.3. Root Dry Weight (g)
The results of the study showed that no significant difference at (p< 0.05) in root dry weight of seeds collected from different provenance. However, at 2 MAP and 3 MAP, seeds obtained from the North, South and East regions were significantly (p < 0.05) different in mean dry weight from seeds obtained from the Western area. Seeds obtained from the southern region had the highest mean dry weight (2.40g) followed by seeds from the East (2.33g) whilst seeds from the West had the lowest mean dry weight (1.4g).The seeds obtained from the east had the highest mean root dry weight at 3 MAP (5.09g) whilst the lowest mean dry weight was recorded from seeds obtained from the west (2.71g) (Table 5).Table 5. Mean root dry weight of seedlings at 1, 2 and 3 MAP
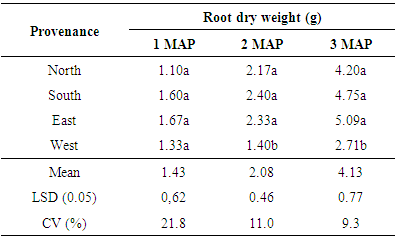 |
| |
|
3.2.4. Shoot Dry Weight (g)
Generally, significant differences were observed in shoot dry weight of seeds obtained from the four provenances. The results showed at 1 MAP there was no significant difference in the shoot dry weight of the seedlings. Significant differences (p < 0.05) were observed between seeds obtained from the East and South at 2 MAP with those obtained from the west. At 3 MAP seeds from the east also had the highest shoot dry weight (7.8g) though not significantly different from seeds sourced from the south and north (Table 6).Table 6. Mean shoot dry weight of seedlings at 1, 2 and 3 MAP
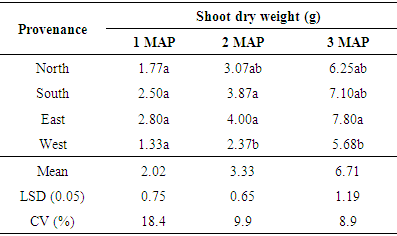 |
| |
|
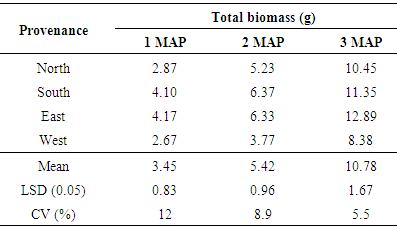
3.2.5. Total Biomass (g)
Significant differences (P< 0.05) were observed in total biomass among the different provenances at 1, 2 and 3 months after planting. The highest total biomass was recorded by the seeds obtained from the east followed by seeds obtained from the south (Table 7). The seeds obtained from the western area had the least total biomass at 1, 2 and 3 MAP (2.67g, 3.77g and 8.38g respectively) Provenance significantly affected shoot dry weight and total biomass of Gmelina seedlings. Seedlings from seeds obtained from the East and Southern regions consistently had higher shoot dry weight and total biomass than seeds obtained from the North and western regions. This could be attributed to the higher average growth rates recorded by seedlings of seeds from the east and south probably due to their large seed sizes. These are areas that favour the growth of forest trees. These results agree with Lauridsen and Kjaer (2002) who stated that seed source had an influence of growth and development of Gmelina arborea.Table 7. Total biomass of seedlings at 1, 2 and 3 MAP
 |
| |
|
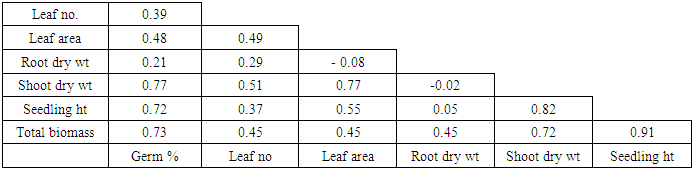
3.2.6. Correlation Matrix of Early Growth Parameters Studied
Results of the correlation analysis showed strong positive correlation (r = 0.91) between seedling height and total biomass. Similarly, a positive correlation was observed between shoot dry weight and total biomass (r = 0.72) while the correlation of root dry weight and total biomass was very weak (r = 0.45). Seedling height had a strong positive correlation with shoot dry weight (r = 0.82).
3.3. Physiological Growth
3.3.1. Average Growth Rate (AGR)
The results of the study indicated that seeds obtained from the west exhibited the least average growth rate both at 2 and 3 months after planting, whilst seeds obtained from the east had the highest average growth rate at 3 MAP (Fig 3). However at 2 MAP, no significant difference (p < 0.05) was observed among the seeds obtained from the North, South and East though significantly different from seeds from the west.Table 8. Correlation matrix of early growth parameters studied
 |
| |
|
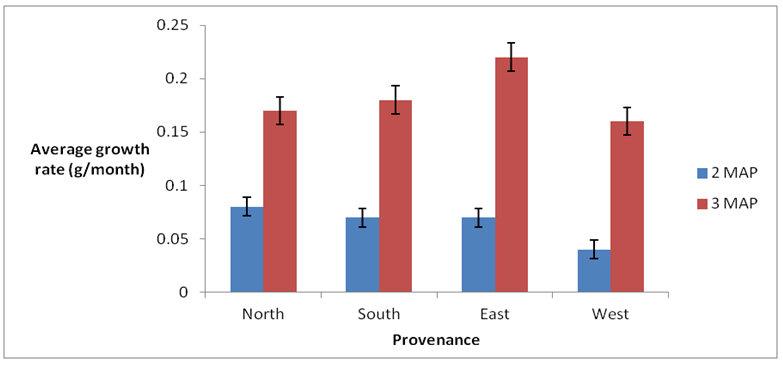 | Figure 2. Average growth rates of seedlings from different provenances |
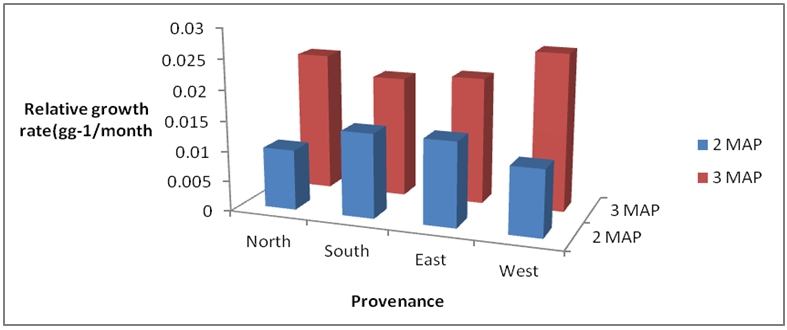 | Figure 3. Effect of Provenance on relative growth rate of Gmelina seeds |
3.3.2. Relative Growth Rate (RGR)
The data on relative growth rate did not show any distinct trend as observed in AGR. RGR of seeds obtained from the east and south had the highest values at 2 MAP whilst at 3 MAP, seedlings of seeds obtained from the west had the highest growth; though not significantly different from seedlings of seeds obtained from the other provenances (Fig 4).
4. Conclusions
From the results of the study, the following conclusions can be made: Seed size is a major parameter for prediction germinability of Gmelina. The best seed sizes to use by tree planters and other stakeholders were the large sized seeds because of their fast germination. Large and heavier seed sizes are recommended as they will promote early maturity of Gmelina arborea for sundry purposes such as pulp for paper production, timber, fuel wood, etc.Seeds obtained from forest plantations in the east and south perform better in terms of early growth than seeds obtained from other forest plantations in the country. Higher rainfall in the east could be responsible for the production of healthy and vigorous seeds of Gmelina. Eastern and Southern seed sources/ provenances be preferred. This study might enable the people growing Gmelina seedlings to produce larger seedlings by pre- selecting and planting large and medium size seeds.
ACKNOWLEDGEMENTS
Acknowledgements go to the staff of Department of Forestry, Njala University for the assistance in conducting the experiment and collection of data. The authors also wish to thank Mr Ishmail Jalloh and Titus Quee for assisting in data collection.
References
| [1] | J. Evans. Plantation Forestry in the Tropics. Oxford Press, UK. 472p. experimental evidence. Functional Experiment 8. 205-214. 1986. |
| [2] | M. A. Ogigirigi, Indigenous fruit tree species of Nigeria for plantation Establishment. Unpublished. 45p on Plant Genetic Resoures pp6-10. 1989. |
| [3] | K. Kadambi, Silviculture and Management of Gmelina. Bulletin 24, School of Forestry. Stephen F. Austin State University, Nachagodoches, Texas, United State of American, 95. 1972. |
| [4] | O Oni, and S.O. Bada, Effect of seed size on seedlings vigour in Idigbo (Terminalia ivorensis. A. Chev). Journal of Tropical Forest Sciences, 4 (3): 218. 1992. |
| [5] | L.A Adegbehin, l. O Abayomi. and L.B. Nwaigbo Gmelina arborea in Nigeria, Agricultural Science 3 (2), 981. 1988. |
| [6] | P. W Owoh, M. O Offiong, S. I. Udofia, and V. U Ekanem. Effects of seed size on germination and early morphological and physiological characteristics of Gmelina arborea Roxb. African Research Review, 5(6), Serial No. 23, 422 – 433. 2011. |
| [7] | S K. Suri Analytical study of teak provenance test in North Raipur Division of Madya Pradesh. The Indian Forester, 110: 345-363. 1984. |
| [8] | P Shiv Kumar and A C Banerjee. Provenance trail of Acacia nilotica. J Tre Sci 5(1): 53-56. 1986. |
| [9] | E.B Lauridsen, and E.D Kjaer, Provenance research in Gmelina arborea Linn., Roxb. A summary of results from three decades of research and a discussion of how to use them. International Forsetry Review 4 (1). 2002. |
| [10] | W.S Dvorak World View of Gmelina arborea: Opportunities and challenges. In Recent advances with Gmelina arborea (eds. W.S. Dvorak, G.R Hodge, W.C Woodbridge and IL Romero) CD – ROM, CAMCORE, North Carolina State University, Raleigh, Ne. USA. 2003. |
| [11] | FAO. A guide to forest handling with special reference to the tropics. FAO Forestry paper 20/2. Food and Agriculture Organization of the United Nations. Rome. Compiled by R.L Willan. ISBN 92-5-102291-7. 1987 |
| [12] | R.T. Odell, J.C Dijkerman, W Van Vuure, S.W Melsted, P.M Sutton, and R Miedema. Characteristics, classification and adaptation of soils in selected areas in Sierra Leone, West Africa. Bulletin 748, Agricultural Experiment Station, College of Agriculture. University ofIllinois at Urna- Champaign, Bulletin 4, Njala University College, University of Sierra Leone. p194. 1974. |
| [13] | D.S.K Jusu. (The socio economic impact of household farmers in Njala, Kori Chiefdom. Unpublished student Project. 1990. |
| [14] | M.O. Offiong, Variation in Growth and Physiological Characteristic of Xylopia aethopica (DUNAL). A Rich from Akwa Ibom and Cross River States. Unpublished Ph.D Thesis. Department of Forest Resources Management University of Ibadan, Ibadan 255pp. 2008. |
| [15] | N Khan, M.D Faridullah and L. Uddin agronomic characters of groundnut (Arachis hypogaea L.) genotypes as affected by nitrogen and phosphorus fertilization under rainfed condition. Electronic Journal of Environmental Agricultural and Food Chemistry, 8(1): 61 – 68. 2009. |
| [16] | B.Z, Zaidman, M Ghanem, and Y Vaknin, Effects of seed weight on vigor and early seedling growth of Jatropha curcas, a bio diesel plant. Seed Science and Technology (38) 757-766. 2010. |
| [17] | J.E Gonzalez Effect of seed size on germination and seedling vigor of Viralakoschy, Warb Forestry Ecology and Management (57): 275 – 281. 1993. |
| [18] | Boot, R. G. A. (1996). The Significance of Seedling Size and Growth Rate of Tropical Forest Tree Seedlings for Regeneration in Canopies Opening. Swaine, M. D. (ed.) The Ecology of Tropical Forest Tree Seedlings. MAB UNESCO Series Vol. 17, pp. 267-284 Parthenon, Paris Press, India. |





 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML






