-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2017; 7(1): 23-27
doi:10.5923/j.ijaf.20170701.04

Leptocybe invasa and Its Effects on Young Plantations of Commercial Eucalyptus Species in Tanzania
1Department of Forest Utilization, Tanzania Forestry Research Institute, Morogoro, Tanzania
2Department of Wood Utilization, Faculty of Forestry & Nature Conservation, Sokoine University of Agriculture, Morogoro, Tanzania
Correspondence to: Revocatus Petro, Department of Forest Utilization, Tanzania Forestry Research Institute, Morogoro, Tanzania.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

A study was conducted to examine the effects of Leptocybe invasa infestation on young plantations of three Eucalyptus species grown in Coastal agro-ecological zone of Tanzania. A total of 12 trees from each species aged six years were sampled. Diameter at breast height (Dbh) and total height for each sampled tree were measured. Results showed that the mean Dbh of infested trees were reduced by 2.1%, 7.8% and 13.6% while heights were reduced by 9.5%, 6.6% and 3.8% compared to uninfested E. tereticornis, E. camaldulensis and E. saligna respectively. The mean basal area of infested trees was reduced by 17.1%, 16.4% and 24.5% and mean volume were reduced by 16.1%, 17.8% and 23.1% compared to uninfested E. tereticornis, E. camaldulensis and E. saligna respectively.
Keywords: Leptocybe invasa, Effect on growth, Eucalypts trees, Tanzania
Cite this paper: Revocatus Petro, Said Iddi, Leptocybe invasa and Its Effects on Young Plantations of Commercial Eucalyptus Species in Tanzania, International Journal of Agriculture and Forestry, Vol. 7 No. 1, 2017, pp. 23-27. doi: 10.5923/j.ijaf.20170701.04.
Article Outline
1. Introduction
- Leptocybe invasa Fisher & La Salle (Hymenoptera: Eulophidae) is a new gall forming invasive wasp, commonly called blue gum chalcid, presumed to have originated from Australia [1]. It was first recorded in the Middle East in 2000 causing infestation on Eucalyptus camaldulensis [1, 2]. Currently, the wasp has subsequently spread throughout many countries in the Mediterranean basin, Africa, Asia, Europe, South America (Brazil), and North America (USA – Florida) (Figure 1) where it is wreaking havoc on Eucalyptus plantations and nurseries [1, 3-8]. However, L. invasa is not considered a pest in Australia where eucalypts are indigenous, suggesting that the natural enemies in its native range keep the population level of the pest below detectable levels [9-11]. In non-native eucalypt forests throughout the world, the wasp poses a serious threat by causing severe defoliation and degeneration of eucalypt seedlings, coppiced shoots and young trees induced by gall formation [1, 9, 12]. Gall formation by L. invasa on petiole and leaves (mainly mid-ribs) of eucalypts causes deformation of terminal shoots and leaves and results in quicker abscission of leaves and drying up of shoots. Severely infested eucalypts show a gnarled appearance, stunted growth, dieback and sometimes tree death [1, 11-13]. Eucalypt seedlings and trees of less than six years old appear to be the most severely affected by attack of L. invasa [10, 14, 15]. In 2007, more than 20,000 hectares (ha) of two year old eucalypts were reported to be affected by gall formation in the southern States of India [16]. Again in India, 2.5 million ha of eucalypts were expected to lose 25% of wood production annually resulting in an annual loss of US$ 20 million due to heavy damage of L. invasa [17]. In Vietnam and India, it was reported that clones were seriously damaged by L. invasa in many nurseries and young plantations and it was becoming increasingly difficult to find seedlings to establish new plantations [10, 18]. In Israel, Thailand and China, planting of E. camaldulensis was stopped because of extensive attacks by the wasp [10]. In Sri Lanka, 4 ha of the coppiced E. camaldulensis trees were damaged by L. invasa where the majority (62.5%) of the trees had low infestation while 10% had heavy infestation [11].
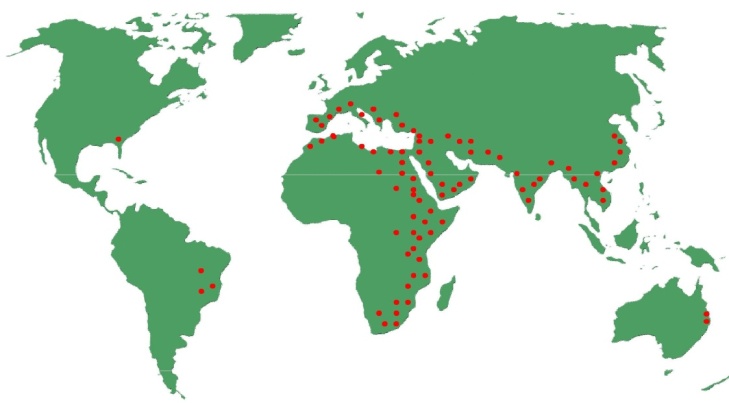 | Figure 1. Global distribution of Leptocybe invasa. Souce; [11] |
2. Materials and Methods
2.1. Description of Study Area
- The study was conducted in private woodlots located in Kibaha district, in the Coastal agro-ecological zone of Tanzania. The district lies between latitude 6.42° and 7.03° South and longitude 38.2° and 38.5° East [24]. The area has bimodal rainfall pattern short rains falling between November and December and long rains between March and May. The mean annual rainfall ranges between 800 and 900 mm, falling on an average of 81 days per year. Average temperature ranges between 23°C and 27°C being highest (33°C) in January and lowest (18°C) in July. Mean annual relative humidity ranges between 53% and 65%, being highest in April and lowest in August and September [15, 22].
2.2. Sampling and Collection of Study Materials
- Purposively sampling method was employed whereby eucalypts stands of six year old were selected for study due to the fact that L. invasa infestation is more severe on young trees [1]. Data were collected from three Eucalyptus species namely E. camaldulensis, E. tereticornis, and E. saligna. The sampled trees (infested and uninfested) of the same species were selected from the same stand with the same age, spacing and management practices. Eucalypt trees were considered as uninfested if there were no any visible galls on shoots/leaves and infested if galls were visible in more than 50% of total shoots. A total of 12 trees (six infested and six uninfested) from each Eucalyptus species were sampled for the study. Dbh for each sampled tree was measured using a calliper while total height was measured using Suunto hypsometer.
2.3. Data Analysis
- Basal area and total tree volume of both infested and uninfested trees were determined according to [25] (equation 1) and [26] (equation 2) respectively. Data were analysed statistically using Excel computer software. T-test was employed to determine significant differences in mean Dbh (cm), total height (m), basal area (m2) and volume (m3) between the means of infested and uninfested trees.
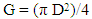 | (1) |
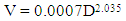 | (2) |
3. Results and Discussion
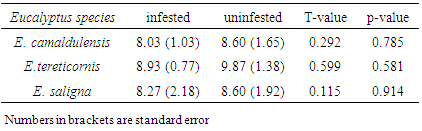
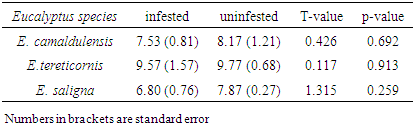
3.1. Diameter and Height Growth Variation between Infested and Uninfested Eucalypt Trees
- Results showed that the mean Dbh and height of all infested trees were relatively lower than uninfested ones (Tables 1 & 2) though their differences were not statistically significant (P>0.05). The mean Dbh of infested E. camaldulensis, E. tereticornis and E. saligna were reduced by 7.8%, 2.1% and 13.6% respectively. Similarly, the heights of infested E. camaldulensis, E. tereticornis and E. saligna were reduced by 6.6%, 9.5% and 3.8% respectively. The recorded growth reduction of infested eucalypts is due to the fact that L. invasa inflicts severe damage by inducing galls mainly on rapidly growing parts like shoots, young stems, petioles or midribs of leaves which form an ideal breeding site for the wasp [10]. Severe damage of growing parts caused by gall forming insects causes loss of stored food in a tree and changes production regulators, which affect photosynthesis and hence cause growth loss in infested plant [1, 27]. The results of this study are relatively lower to a reduction of 53% and 25% of height and diameter respectively of E. regnas saplings when infested by Chrysophtharta bimaculata for a period of eight years [28]. Similarly, results of E. camaldulensis and E. saligna in this study were contrary to a higher growth reduction of height than that of diameter due to insect pests infestation [23, 28].
|
|
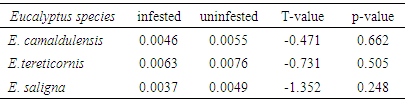
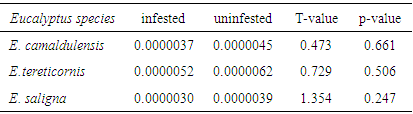
3.2. Mean Basal Area and Volume Variation between Infested and Uninfested Eucalypt Trees
- Results showed that the mean basal area and volume of all infested trees were relatively lower than uninfested ones (Tables 3 & 4) although their differences were not statistically significant (P>0.05). The mean basal area of infested trees were reduced by 16.4%, 17.1% and 24.5% compared to uninfested ones for E. camaldulensis, E. tereticornis and E. saligna respectively. Similarly, the mean volume of infested trees were reduced by 17.8%, 16.1% and 23.1% compared to uninfested ones for E. camaldulensis, E. tereticornis and E. saligna respectively. The reduction of basal area and volume in infested trees shows that L. invasa infestation results in loss of productivity. The recorded loss in productivity in this study is due to the fact that L. invasa infestation causes severe injury to young foliage of Eucalyptus species by inducing galls which become a major constraint in growth and wood production [1]. In another study, a reduction of 16% in basal area of E. globulus was reported when mildly infested by Perga affinis (Sawfly) for a period of four years [29]. They further reported a reduction of 31% in basal area of the same species when severely infested by P. affinis. When assessing the impact of leaf defoliators in Eucalyptus plantations, a reduction of 30% of wood volume of E. regnans saplings when infested by C. bimaculata for a period of eight years was reported [28]. In Tasmania, it was reported that, when E. regnans trees are repeatedly damaged by Chrysomelid leaf beetles results to the loss of about 52% of their basal area increment [30], while other authors reported defoliation in trials running for more than 10 years, that repeated defoliation episodes reduced overall economic viability of plantations significantly [31].
|
|
4. Conclusions and Recommendations
- The study has shown that L. invasa infestations affect the productivity of commercial eucalypt trees. Based on the reduction of diameter, basal area and volume of all three eucalypt species, it is clear that the effect of L. invasa on growth was higher on E. saligna than on E. camaldulensis and E. tereticornis. These variations in susceptibility indicate an opportunity for selecting eucalyptus species for resistance against the pest. It is therefore recommended to plant resistant or less susceptible eucalypt species in order to avoid much loss in productivity.
ACKNOWLEDGEMENTS
- We are grateful to the Tanzania Commission for Science and Technology (COSTECH) for financing this study. We are grateful to I. Hussein and A. Shekiombo of Tanzania Forestry Research Institute (TAFORI) for their assistance during data collection.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML