-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2016; 6(6): 197-205
doi:10.5923/j.ijaf.20160606.01

Effects of Microbial Biomass and Activity on Carbon Sequestration in Soils under Different Planted Forests in Chittagong, Bangladesh
Md. Mamunur Rasid, Nasrin Chowdhury, Khan Towhid Osman
Department of Soil Science, University of Chittagong, Chittagong, Bangladesh
Correspondence to: Nasrin Chowdhury, Department of Soil Science, University of Chittagong, Chittagong, Bangladesh.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Microbial biomass, activity and ecophysiological indices together with important physical and chemical properties were studied in soils under four different planted forests (Acacia auriculiformis, Artocarpus chaplasha, Dipterocarpus turbinatus and Lagerstroemia speciosa) in Chittagong, Bangladesh. The principal objective of the study was to investigate microbial activity related to carbon sequestration under different tree species. The mean values of pH, total nitrogen, organic carbon, microbial activity and microbial biomass carbon varied from 4.49 to 4.68, 0.9 to 1.9 mg kg-1, 6.4 to 10.6 mg kg-1, 0.06 to 0.13 mg CO2 g-1d-1 and 736.82 to 844.44 µg C g-1 respectively. Plant biomass as well as organic carbon content, microbial biomass and activity in soil was the highest in Artocarpus chaplasha. There were strong positive correlations between plant biomass and almost all the soil properties which suggested that plant species affected carbon transformations in the studied soils. Significant difference (P < 0.05) in organic carbon content and microbial activity was observed in soils under Acacia auriculiformis from soils under all other species. The ratio of microbial biomass carbon to soil organic carbon (qMic) was the highest and basal respiration and microbial biomass carbon ratio (qCO2) was the lowest in soils under Acacia auriculiformis and the variation was significant with soils of other species. Therefore, Artocarpus chaplasha the indigenous species of Bangladesh has greater potentiality to sequester carbon in soils than other three species, and Acacia auriculiformis the exotic species has the least contribution to sequester carbon in these soils.
Keywords: Soil organic carbon, Biomass carbon, Soil respiration, Forest, Ecophysiological indices
Cite this paper: Md. Mamunur Rasid, Nasrin Chowdhury, Khan Towhid Osman, Effects of Microbial Biomass and Activity on Carbon Sequestration in Soils under Different Planted Forests in Chittagong, Bangladesh, International Journal of Agriculture and Forestry, Vol. 6 No. 6, 2016, pp. 197-205. doi: 10.5923/j.ijaf.20160606.01.
Article Outline
1. Introduction
- There is a strong interest in stabilizing the atmospheric abundance of carbon dioxide (CO2) and other greenhouse gasses to mitigate the risks of global warming [1-4]. CO2 sequestration is an important strategy of lowering CO2 emissions from point sources through natural and engineering techniques [5]. The global carbon (C) cycle is heavily dependent on microbial communities that fix atmospheric C, promote plant growth and transform organic material in the environment. Most plant production (80-90%) enters the soil as dead wood, leaves, exudates, roots and exudates. Soil microbes are the main agents of its breakdown because they produce enzymes capable of degrading recalcitrant plant-derived compounds such as lignin and cellulose. As a result, a large proportion of soil respiration is sourced from the activity of heterotrophic microbes. The microbial contribution to soil carbon storage is directly related to microbial community dynamics and the balance between formation and degradation of microbial by-products. The amount of substrate C that is incorporated into microbial biomass and eventually transferred into the microbial derived organic matter pool is determined by the size of the microbial biomass and microbial growth efficiency. Microbial biomass acts as an intermediary controlling the type and quantity of C that can be actually removed from the atmosphere and the time scales that C stay sequestered. Because the C cycle, especially CO2, plays a great role in global climate change, understanding soil microbial activity and biomass will improve understanding of climate change and long-term C storage in soils. On the other hand, microbial biomass can also act as potential indicator of C sequestration as they can detect tillage and crop rotation effects on soil earlier than total organic carbon or nitrogen measurements in soil [6].Forestry appears to offer a relatively low-cost approach to sequestering C. Plants acts as a medium for transfer of atmospheric C into the soil in the form of C-containing compounds. The amount of C stored in forest soil is about 124 Pg [7]. C sequestration in forest soils depends on the balance between C inputs through photosynthesis and outputs through soil microbial respiration [8]. It is, therefore, evident that forests and land-use plays an important role in the global C cycle and that a clear understanding of this role is a vital component of attempts to understand and combat the causes and consequences of climate change. Evergreen and mixed evergreen tropical rain forests once existed in the southern hills of Bangladesh. During past few decades, the original dense natural cover has largely been removed. Currently, there are 12777 sq km reserve forest land and 3268 sq km state forest land corresponding to 8.65 and 2.21 percent respectively of the total land area of Bangladesh [9]. With the fast rates of deforestation afterwards, the need for bringing denuded areas under rapid and effective cover became more essential. Chittagong is situated in the hilly region of Bangladesh. Bangladesh Forest Department maintains the afforestation and reforestation programme in this area. Afforestation and reforestation should be a useful means by which C could be stored in the various components of a forested ecosystem [10]. Soil CO2 flux can be varied with forest productivity in different ecosystems [11] and silvicultural treatments. Forest management activities have different effects on soil C sequestration [12]. The influences of forest management practices on soil C dynamics were studied elaborately [13]. However, little is known about the effects of forest stand on C sequestration on the perspective of soil microbial processes.Since soil microorganisms are the primary agents of the soil ecosystem responsible for litter decomposition, nutrient cycling and energy transfer processes, a research programme was designed to investigate the microbial activity and biomass in relation to the potentiality of C sequestration in soils under Acacia auriculiformis, Artocarpus chaplasha, Dipterocarpus turbinatus and Lagerstroemia speciosa forests of Chittagong and to obtain basic information for long-term monitoring as well as possible short-term effects. This study is critical to accurately predict effects of forest management on the C cycle and to develop appropriate forest management strategies aimed at reducing atmospheric CO2 concentrations.
2. Materials and Methods
2.1. Study Area
- The study was conducted in some forest plantations on moderately steep slopes (10-12%) of medium (<50 m) hills in the University of Chittagong campus in Hathazari Upazila of Chtttagong district, Bangladesh (Figure 1). The geographical position is 22°27'30'' and 22°29'0'' North latitudes and 91°46'30'' and 91°47'45'' East longitudes. These soils belong to Brown Hill Soils according to the General soil type which are equivalent to Dystric Eutrochrepts according to USDA Soil Taxonomy [14] and were formed from tertiary unconsolidated sediments.
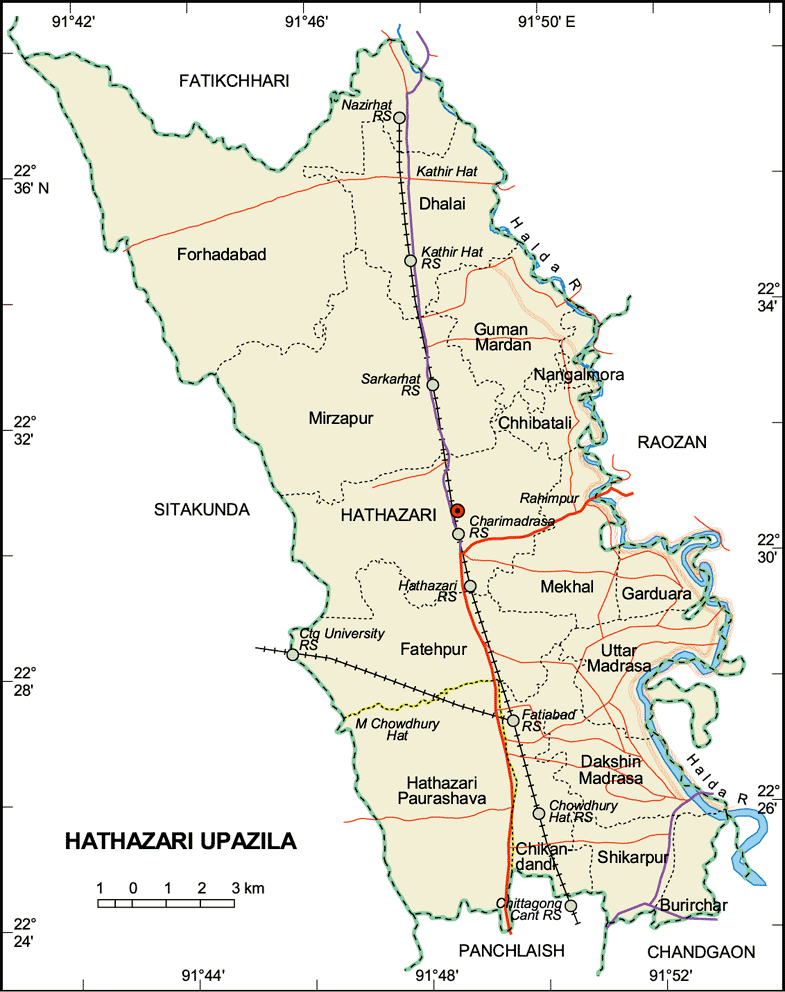 | Figure 1. Location of the study area (University of Chittagong, Chittagong, Bangladesh) [15] |
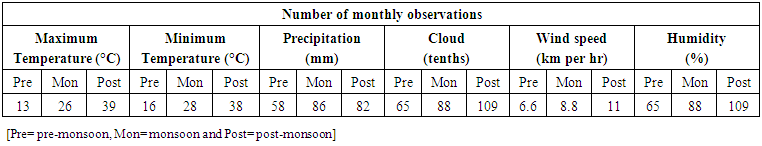 | Table 1. Meteorological data of the study area (University of Chittagong, Chittagong, Bangladesh) [18] |
2.2. Sampling Plots and Sampling Design
- Some of the data were collected through physical measurement in the field and from plantation records of the Institute of Forestry and Environmental Science of University of Chittagong. These plantations were 30 years old. Canopy coverage of the sites was almost 90% and undergrowth consisted of different kinds of herbs and shrubs. Nine sample plots (10 m x 10 m) were distributed over the entire planted area of each species and soil samples were collected from each plot in April, 2014. Girth diameter of all the trees in a plot was measured by using a diameter tape. Total height was measured using a Spiegel Relaskope. Complete Randomized Block Design (CRBD) was followed for taking samples from the sample plots.
2.3. Processing of Soil Samples
- Soil samples were taken from surface 0-15 cm in polythene bags and brought into the laboratory. Each soil sample was divided into two sub-samples, one for physical and chemical analysis, and the other was used for soil microbial analysis. The soil samples for chemical and physical analysis were air dried, passed through a 2 mm sieve and were preserved in plastic pots before analysis.
2.4. Analysis of Physical and Chemical Properties of Soil
- The particle size analysis of soils was carried out by the hydrometer method as described in [19]. Bulk density was estimated by core method [20]. Maximum water holding capacity (WHC) of the soils was measured volumetrically. Soil pH was determined with standardized pH meter in a suspension having soil: water ratio of 1:2.5 [21]. Cation exchange capacity (CEC) of soils was determined with IN NH4OAC buffered at pH 7.0 according to the method of Jackson [22]. Total nitrogen (TN) content of soils was determined by micro-Kjeldahl digestion and distillation method as described in [23]. Organic C content in soils was determined by the wet oxidation method (chromic acid digestion) of Walkley and Black [24] as described by Jackson [22].
2.5. Analysis of Soil Microbiological Properties
- Viable aerobic bacteria and fungal cell numbers in fresh soils were counted using the dilution plate method as described in [25]. Nutrient agar medium was used for bacteria and potato dextrose agar medium for fungi. Three plates were used for each soil. The plates were incubated at 28°C for 72 h to 120 h and counting were made for forming colonies. Microbial activity was determined by soil respiration, trapping the CO2 in sodium hydroxide (NaOH) which was evolved from the soil during incubation in a closed system [26]. The trapped CO2 was determined by measuring electrical conductivity [27]. For this purpose, 50 g soil (oven dry basis), moistened to 50% of water holding capacity, was placed in 1 liter capacity incubation jars. A 10 ml aliquot of 0.5 M NaOH solution in 50 ml falcon tubes were placed in each jar as the CO2 trap. A falcon tube with water was added into the jar to maintain the soil moisture. Jars were made air tight immediately. Two jars with 0.5 M NaOH but without soil were used as controls. All jars were incubated at 25°C. The CO2 absorbed in traps were analyzed at each days of NaOH placement. Each time fresh NaOH solution (10 ml) was replaced to trap CO2 for the next days. In this method, CO2 evolved from each sample was calculated as the difference between the initial and the CO2 concentration after each measurement period. Basal respiration rate was calculated based on cumulative CO2 evolution over the 11 day period. The substrate induced respiration (SIR) allows the estimation of the amount of C held in non-resting, living microorganisms in soil sample. The initial respiratory response to added glucose, recorded before any development in existing soil microflora could be viewed as an index of the existing soil microflora [28]. Soil respiration was assessed as stated above. The substrate induced respiration values were transformed to biomass C using the formula as described by West and Sparling [29] as follows:
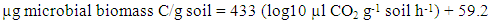 | (1) |
2.6. Eco-physiological Indices
- The mineralization quotient (qM) indicating the fraction of total organic C mineralized calculated from basal respiration and soil organic C ratio (μg CO2-C/Corg d-1) and was expressed as percent (%) to describe the percent of organic C evolved as CO2 d-1.
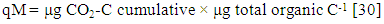 | (2) |
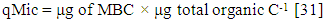 | (3) |
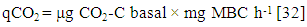 | (4) |
3. Results
3.1. Tree Species
- The mean data for foliar mean density of tree numbers, height, diameter at girth, above ground biomass and below ground biomass in four tree species is summarized in Table 2. The maximum tree height was found in A. chaplasha (17 m) followed by A. auriculiformis (15.67 m), D. turbinatus (14.00 m) and L. speciosa (13.67 m). Mean diameter at girth of exotic tree species (A. auriculiformis) showed lower volumetric growth (72 cm) than indigenous forest tree species. Biomass of trees per unit hectare of the indigenous forest tree species was also higher than exotic tree species. The highest biomass content was found in A. chaplasha (294.67 t ha-1) followed by L. speciosa (284 t ha-1) and D. turbinatus (279 t ha-1) all of which are pure indigenous species for Bangladesh.
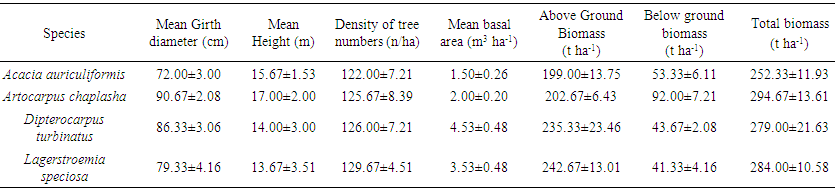 | Table 2. Volume of tree species in unit area, above ground, below ground and total biomass of tree species in study area |
3.2. Soil Chemical and Physical Properties
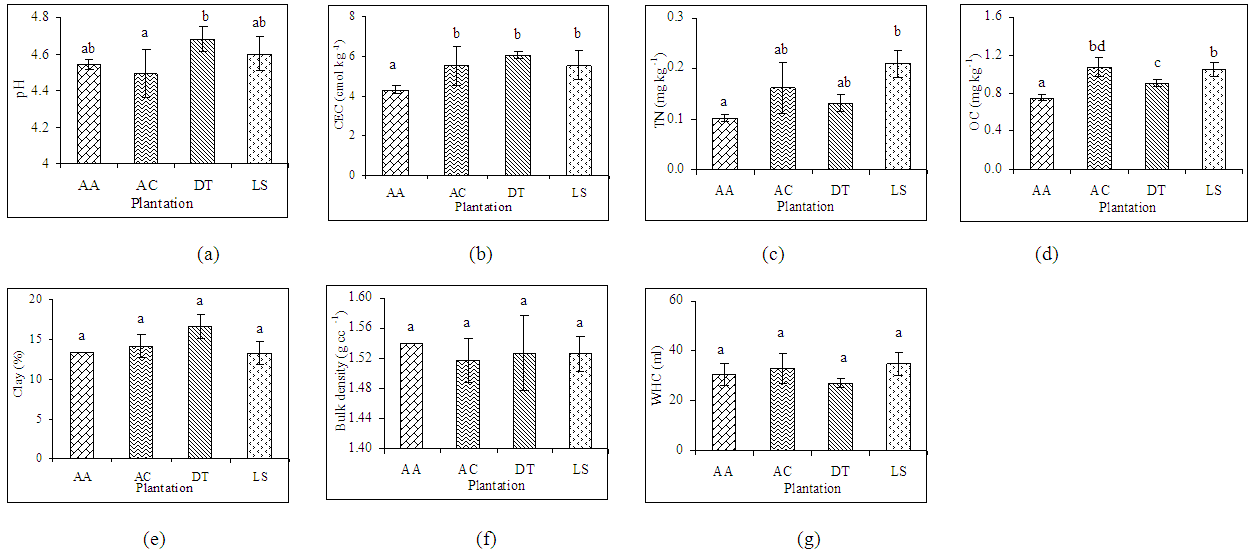
- The studied soils were slightly to strongly acid soils. Soil pH, CEC and TN content varied from 4.49 to 4.68, 4.31 to 6.05 cmol kg-1 and 0.9 to 2.30 mg kg-1 respectively (Figure 2). The CEC was significantly low in soils under A. auriculiformis than soils under indigenous species (Figure 2b). There were significant (P<0.05) differences between soils under tree species for concentrations of organic C. Organic C content in soils under A. auriculiformis varied from 6.4 to 8.7 mg kg-1 and the mean value was 7.5 mg kg-1 (Figure 2d). Mean organic C content was the highest in soils under A. chaplasha (10.8 mg kg-1) than the soils under other plant species (Figure 2d). Soil texture was sandy loam everywhere. The bulk density and water holding capacity ranged from 1.52 to 1.54 g cm-3 and 29.98 to 34.75% respectively. Correlation analysis revealed significant relationship of different plant growth parameters on chemical and physical properties. Total biomass of tree species strongly affected organic carbon (r= 0.74, p< 0.001), total nitrogen(r= 0.66, p< 0.001) and cation exchange capacity (r= 0.62, p< 0.001) in soils under four planted forests (Table 3).
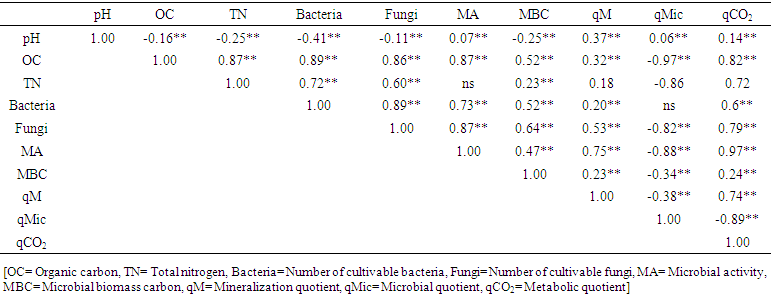 | Table 3. Pearson’s correlation coefficients between soil chemical, physical and microbiological properties |
3.3. Soil Microbial Properties
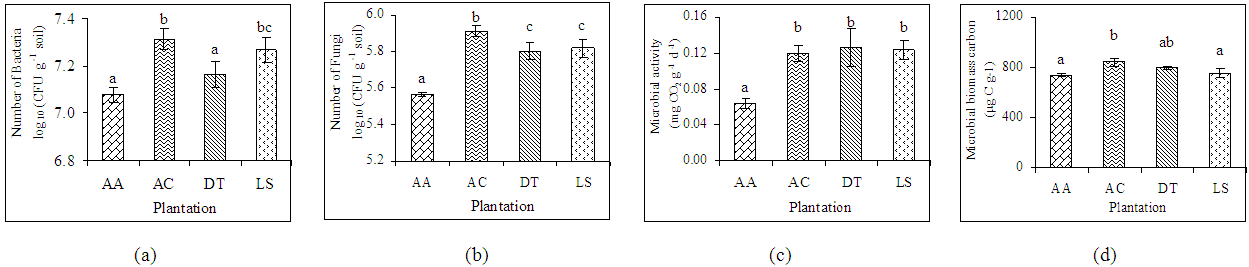
- Soil microbial properties under all the plant species differed significantly with each other (Table 3). Microbial parameters were found higher in indigenous tree species than the exotic species A. auriculiformis. Within the indigenous tree species A. chaplasha contained higher number of cultivable bacteria and fungi than L. speciosa and D. turbinatus (Figure 3 a,b). Microbial C content was also high in soil under A. chaplasha (844.44 µg C g-1). The rate of basal respiration varied from 0.06 to 0.07 mg CO2 g-1day-1, 0.11 to 0.13 mg CO2 g-1 day1, 0.10 to 0.14 mg CO2 g-1day-1 and 0.11 to 0.13 mg CO2 g-1day-1 with a mean value of 0.06, 0.12, 0.13 and 0.12 mg CO2 g-1 day-1 in soils under A.auriculiformis, A. chaplasha, D. turbinatus and L. speciosa respectively (Figure 3). Fungi in forest floor were more significantly affected than bacteria by all the plant growth parameters. Total biomass of plantations strongly affected number of bacteria (r= 0.62, p< 0.001), number of fungi (r= 0.72, p< 0.001), microbial biomass C (r= 0.695, p< 0.001) and microbial activity (r= 0.669, p< 0.001) (Table 3).
3.4. Eco-physiological Indices
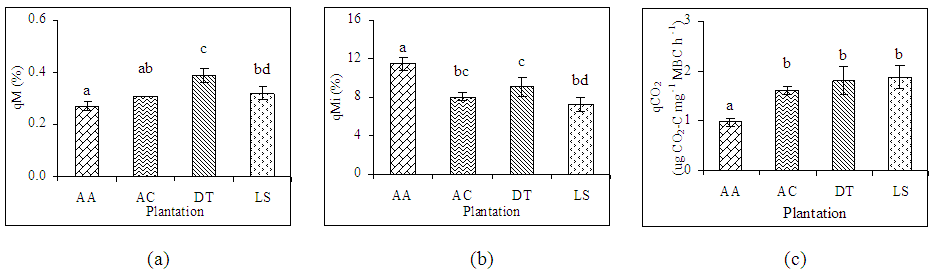
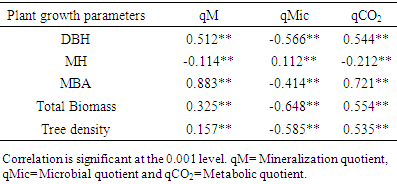
- Mean mineralization quotient was the lowest in soils of A. auriculiformis (0.27%) and the highest in soil of D. turbinatus (0.39%) (Figure 4). Mean microbial quotient was the highest in soils of A. auriculiformis (11.47%) and the lowest in soils of L. speciosa (7.22%). The qMic in soils of A. auriculiformis had significant difference with the soils of other sites (Figure 4c). The variation of qMic in soils of D. turbinatus and L. speciosa was also significant. Values of metabolic quotient (qCO2) in soils of A. auriculiformis, A. chaplasha, D. turbinatus and L. speciosa varied from 0.90 to 1.09, 1.56 to 1.72, 1.45 to 1.98 and 1.56 to 2.03 (µg CO2-C mg-1 Cmic h-1) with a mean value of 0.98, 1.61, 1.80 and 1.87 (µg CO2-C mg-1 Cmic h-1) respectively. Mean value of qCO2 was the highest in soils of L. speciosa and the lowest in soils of A. auriculiformis (Figure 4c). Total biomass of plantations strongly affected qMic (r= -0.648, p< 0.001 (Table 5).
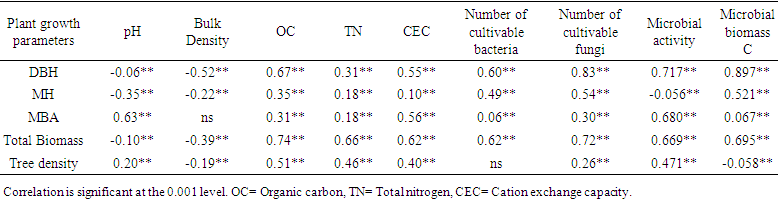 | Table 4. Pearson’s correlation coefficients between different plant growth parameters of tree species in the study area in unit area and soil properties |
|
4. Discussion
4.1. Plant Species
- Results show that indigenous species can sequester more organic C than exotic species. Among the indigenous species A. chaplasha had the highest biomass content (299 t ha-1). Therefore, it has the ability to store more C and act as sink than exotic species in plantations. Akter et al. [33] and Alamgir and Al-Amin [34] found that mean organic C was the highest for indigenous species than exotic species in Chittagong University campus. Higher amounts of plant residue inputs in soil might either accelerate decomposition and reduce C storage through decreased metabolic efficiency or enhance decomposition of existing soil C. Alternatively or in parallel, higher amounts of plant residue inputs could enhance C storage through increased mass of dead plant material accumulation over time. The observed increase in C storage with plant species therefore either reflects higher primary production or longer persistence of plant-derived organic materials due to slower decomposition. Increased plant residue inputs can also provide more substrate for soil microorganisms, resulting in a more active and more abundant microbial community in study area.
4.2. Soil Chemical and Physical Properties
- The C storage in forest soils is affected by forest type, and site quality [35] and management practices, such as fire, clear felling etc. There were significant differences between soil chemical and physical properties under the four different tree species (Table 3). A. auriculiformis contributes the lowest content of organic C in soils which is 1.5 times less as compared with A. chaplasha and L. speciosa (Figure 2d). Mean organic C content was the highest in soils under A. chaplasha due to return of greatest quantity of litter to the soil. Deciduous tree species usually have higher-quality (low C:N) foliar litter than do evergreen tree species [36]. Litter from different tree species decomposes at different rates [37, 38, 39], thereby influencing soil C accumulation rates [40].
4.3. Soil Microbiological Properties
- Individual plant species are known to influence soil microbial activity and nutrient cycling through the quality and quantity of organic matter return to the soil [41]. Basal respiration represented the mineralization of native organic substances in the soil samples. Mean respiration rate indicating the microbial activity and Microbial biomass C as well as number of cultivable bacteria and fungi were the highest in soil under A. chaplasha. On the other hand, these parameters were the lowest in soils of A. auriculiformis.The levels of microbial biomass C differed between soils under indigenous and exotic species (Figure 3). This indicates that the maintenance of native vegetation ensures the best conditions (plant biomass and N level), with a positive influence on the development and establishment of the soil microbiota [42] (Table 4). Though microbial population was higher in soils of L. speciosa than D. turbinatus, microbial biomass C was higher in soils under D. turbinatus as the fungi to bacteria ratio was higher in soils of D. turbinatus (0.81) than L. speciosa (0.80). Menyailo et al. [43] and Sinha et al. [44] reported that the secondary broadleaved forest had higher soil microbial biomass C and N than the Cunninghamia lanceolata plantations indicating that tree species affected soil microbial processes.
4.4. Eco-physiological Indices
- Microbiological parameters were correlated in many studies as an index [45]. The mineralization quotient (qM), microbial quotient (qMic) and metabolic quotient (qCO2) are have often been used for evaluating the microbial ecophysiology implying an interlinkage between cell-physiological functioning under the influence of environmental factors [46]. In this study the responses of different plant species to ecophysiological indices found to be strongly affected (Table 4). Significant changes in qM, or the potential C mineralization means that all the plant species affected the capacity of the soils to store C. Microbial metabolic quotient is a sensitive indicator of the biological activity and substrate quality [31, 47]. The low qMic and the high qCO2 reflect a less efficient use of organic substrates by microbial biomass [46, 30]. Mean microbial quotient ranged form 7.22 – 11.47% and was in the order of A. auriculiformis> D. turbinatus> A. chaplasha> L. speciosa which denotes percentage of metabolically active C is highest in soil under A. auriculiformis and lowest in soil under L. speciosa. Even though the soil under A. auriculiformis had lower organic C and microbial activity, it has a higher microbial quotient as compared to the soils under other species. Higher value of qMic in soil under A. auriculiformis also represents assimilation of C by microbes. In addition, lower rate of decomposition may facilitate higher rate of C assimilation. When the microbial biomass becomes more efficient in the use of the ecosystem resources, less CO2 (per unit of microbial biomass C) is lost through respiration and a higher amount of the C is incorporated into microbial biomass, resulting decrease in qCO2 [48]. The mean value of metabolic quotient varied from 0.98 – 1.87 (µg CO2-C mg-1 Cmic h-1) in the studied soils and was in the sequence of L. speciosa> D. turbinatus> A. chaplasha to A. auriculiformis. This indicates that soil microbes under L. speciosa had least C use efficiency where as soil microbes under A. auriculiformis were utilizing C at peak level. We can therefore suggest that, the C available for microbes has been used to build up more microbial biomass as the significant increase of microbial quotient under A. auriculiformis. A. auriculiformis provided C for microbial growth, but number of cultivable bacteria and fungi, microbial activity and biomass were lower than other plant species in its soil. On the other hand lower rate of mineralization in soils under A. chaplasha (0.31%) than L. speciosa (0.32%) and D. turbinatus (0.39%) along with higher organic C, microbial population indicate that sequestered C was higher in this site.Based on physico-chemical and microbial properties as well as statistical analyses for investigating the potentiality of C sequestration, it can be sum up that carbon dynamics was higher in A. chaplasha than all other species and therefore has greater potentiality to sequester C in soils.
ACKNOWLEDGEMENTS
- The financial support from the University of Chittagong, Bangladesh, is gratefully acknowledged.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML