-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2016; 6(4): 137-141
doi:10.5923/j.ijaf.20160604.01

Response of Guar (Cyamopsis teteragonolopa L.) to Bradyrhizobium Inoculations in Semi-arid Environment
Khalid A. Ibrahim1, 2, Elsadig A. M. Naeim3, Ahmed M. El Naim4, Mohammed A. Elsheikh5
1Department of Soil and Water Sciences, Faculty of Natural Resources and Environmental Studies, University of Kordofan, Elobeid, Sudan
2Prince Sultan bin Abdul Aziz Center for Research & Environmental Studies & Tourism, King Khalid University, Abha, Kingdom of Saudi Arabia
3Deanship of Postgraduate & scientific Research University of Kordofan, Elobeid, Sudan
4Department of Crop Sciences, Faculty of Natural Resources and Environmental Studies, University of Kordofan, Elobied, Sudan
5Department of Soil and Environment Sciences, Faculty of Agriculture, University of Khartoum, Khartoum, Sudan
Correspondence to: Ahmed M. El Naim, Department of Crop Sciences, Faculty of Natural Resources and Environmental Studies, University of Kordofan, Elobied, Sudan.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bio-fertilizers are environment friendly and protect the environment against the pollutant. Afield experiment was conducted in semi-arid environment ”North Kordofan of Sudan” in rainy seasons (2008/09) to investigate the effect of inoculation of Bradyrhizobium on growth and yield of guar (Cyamopsis teteragonolopa L.) in gardoud soil. The treatments used were: un-inoculated control, inoculated with TAL169 and Hi12 (introduced), and other12 strains (Local Isolated Strains). The experiment was arranged in a randomized complete block design with three replications. Estimated Parameters were: fresh and dry weight of shoot and root, plant height, number of fruiting branches, number of nodules, number of pods per plant, 100-seed (g) and total seed yield (ton/ha). The results showed that, the Bradyrhizobium significant increased growth and yield attributes of guar.
Keywords: Guar, Nodulation, Bradyrhizobium, Seed Yield
Cite this paper: Khalid A. Ibrahim, Elsadig A. M. Naeim, Ahmed M. El Naim, Mohammed A. Elsheikh, Response of Guar (Cyamopsis teteragonolopa L.) to Bradyrhizobium Inoculations in Semi-arid Environment, International Journal of Agriculture and Forestry, Vol. 6 No. 4, 2016, pp. 137-141. doi: 10.5923/j.ijaf.20160604.01.
Article Outline
1. Introduction
- Legumes are plants of the pea or bean family. The Leguminosae is one of the largest families of flowering plants with 18,000 species classified into around 650 genera [1]. This is just under a twelfth of all known flowering plants. The Leguminosae constitute one of humanity’s most important groups of plants. Legumes are used as crops, forages and green manures. Many more legumes are local food plants. In addition to those legumes mainly cultivated for human consumption, many yield important fodders, green manures and forages, e.g., Lupinus (lupin), Medicago (alfalfa) and Trifolium (clover). Legumes are utilised for a variety of other purposes including timber, medicine, tannins and gums [2]. Guar, or cluster bean, (Cyamopsis tetragonoloba (L.) Taub) is a drought-tolerant annual legume grown principally in India and Pakistan. Also guar is cultivated in USA, Australia and Africa but in small areas. It can be eaten green like snap bean, feed to cattle or used as green manure [3]. Biological Nitrogen Fixation (BNF) is an efficient source of fixed N2 that plays an important role in land remediation. Interest in BNF has focused on the symbiotic systems of leguminous plants and rhizobia because these associations have the greatest quantitative impact on the nitrogen cycle. Deficiency in mineral N often limits plant growth and as such, symbiotic relationships have evolved between plants and a variety of N2-fixing organisms. The symbiotically fixed N2 by the association between Rhizobium species and the legumes represents a renewable source of N for agriculture. Values estimated for various legume crops and pasture species are often impressive, commonly falling in the range of 200 to 300 kg N ha-1 per year. This underlines the significance of Rhizobium and legume symbioses as a major contributor to BNF. Nitrogen fixation, along with photosynthesis as the energy supplier, is the basis of the soil environment under a constant state of change and, as such, can be relatively stressful for both macro- and micro-organisms [4].Inoculation of appropriate strains of rhizobia to legumes known to enhance yield, but both success and failures at field experiments have been documented [5]. Rhizobium inoculation of faba beans was reported to increase yield and protein content. The effect of inoculation with R. japonicum on nodulation, plant growth, and yield of diverse soybean [Glycine max (L.) Merr.] Cultivars was studied in Nigeria and Tanzania. Local cultivars from Nigeria and Indonesia nodulated with indigenous Rhizobium spp. in Nigeria. Inoculation with several strains of R. japonicum occasionally increased nodule mass of the local cultivars. Large increases in growth and yield were obtained when the cultivars were inoculated [6]. Inoculation with a selected rhizobium strain resulted in a significant increment in nodule number per plant, nodule dry weight and yield of Chickpea [7]. In sub-Saharan Africa, inoculation of soybean (Glycine max (L.) Merrill) with Bradyrhizobium japonicum increased yield from 500 to 1500 kg ha-1 [8]. The inoculated haricot bean in Sudan, showed that a local strain of Rhizobium significantly improved nodulation and usually increased the nitrogen content of plants. Also increases in seed yield were obtained ranging from 20–145 Per cent. Soil inoculation gave better early nodulation than seed inoculation, but the difference diminished in the later stages of plant growth [9]. Musa and Burhan [10] reported that Soybeans in the Gezira Scheme was a poor forage yielder with delayed nodulation and nitrogen fixation. Inoculation of groundnut genotypes with a very effective strain of Rhizobium increased nitrogen fixation and pod yield when the crops were grown in fields well populated with effective strains of Rhizobium [11]. The objective of this research study is to investigate the effect of inoculation with bradyrhizobium strains on growth and yield of guar crop.
2. Materials and Methods
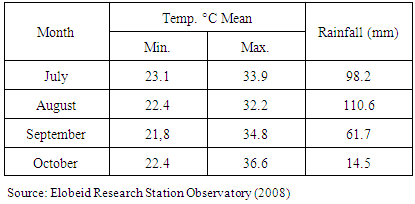
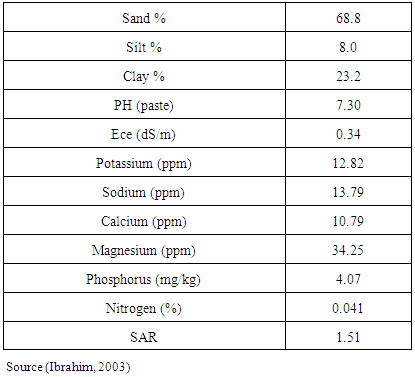
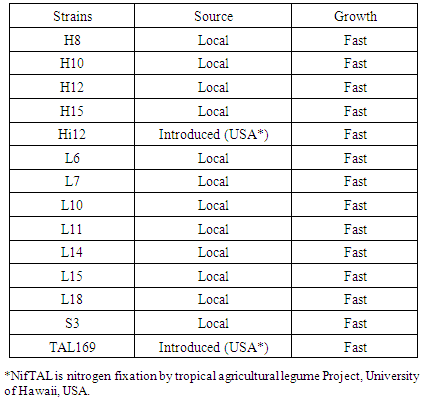
- A field experiment was conducted during the seasons (2008/09) under rain fed, on in North Kordofan State, Sudan, latitude (11° 15 and 16° 30 N) and longitude (27° and 32° E). The climate of the area is arid and semiarid zone. The soil has low fertility. Annual rainfall ranges between 350 – 500 mm. Average maximum daily temperatures varied between 30° and 35°C throughout the year [13]. Physio-chemical properties of gardud soils are shown in Table 1. The experiment was arranged in a randomized complete block design with three replications. The land was prepared and divided to plots 3.0 m x 2.0 m each. Treatments used were: un-inoculated control, inoculated with TAL169 and Hi12 (introduced), and other12 isolated strains (Local). Using local Guar cultivars (Local). The seeds supplied by the ministry of Agricultural and Forestry, Sudan. Seeds Inoculated by Twelve locally isolated strains of Rhizobium sp. (shown in Table 2) provided by Biofertilizers Department, Environment and natural Resources Institute, National Centre for Research, Khartoum, Sudan, in addition to another two introduced strains of Bradyrhizobium sp. (TAL 169 and Hi12) offered by NifTAL Project, Paia, Hawaii, USA. The strains were maintained at 4°C on Yeast Extract Mannitol Agar (YEMA) slopes. Sowing dates were on the July, 13th, 2013 for first season, and on 17th of July for second season. Ten inoculated seeds were put in a hole with space 40 cm between holes and 70 cm between rows immediately after rainfall. Thinning to three plants per hole two weeks later. Sampling began after four weeks from sowing date, and so every four weeks till harvesting. A sample of plants were taken from each plot to carry the parameters. Then placed in a paper bag and transported to the lab to estimate the following parameters:1- Plant height (cm): Plant height (cm). Measured from the ground level to the tip of the plant.2- Number of nodules/plant. Number of nodules was determined by counting the number of differential nodes of the main stem.3- Shoot fresh weight (g)4- Shoot dry weight (g): by weighed dry at 85oC for 24 hours to constant weight.5- Root fresh weight (g)6- Root dry weight (g): by weighed dry at 85oC for 24 hours to constant weight.7- Number of pods per plant8- 100-seed weight (g): 1000-seed weight (g) was determined by counting 1000-seeds at random from each lot of plot four times and weighed by a sensitive balance9- Seed yield (tonlha):
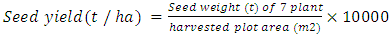
|
|
|
3. Results and Discussion
3.1. Growth Attributes
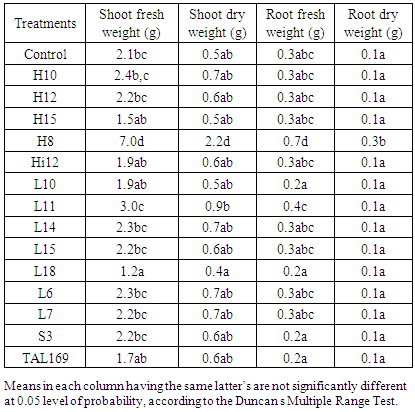
- Inoculation of guar genotype with local and introduced rhizobium strains significantly (p≤0.05) effect the most growth and yield attributes studied (Table 4. 5 and 6). Inoculation increased plant height (Table 4). The strains H10, H8, L10, L11, L18, L6 and L7 increased plant height by 6%, 8%, 31%, 4%, 38%, 15% and 26% respectively (Table 4). Similar were reported by Ibrahim, et al, [20] that inoculation enhanced plant height of guar and. Inoculation of guar genotype with rhizobium strains significantly (p≤0.05) increased shoot fresh weight. While inoculation with strains H10, H12, H8, L10, L18 and L7 significantly (p≤0.05) increased the shoot fresh weight by 2%, 71%, 59%, 45%, 7% and 55% respectively (Table 5).
|
|
|
3.2. Yield Attributes
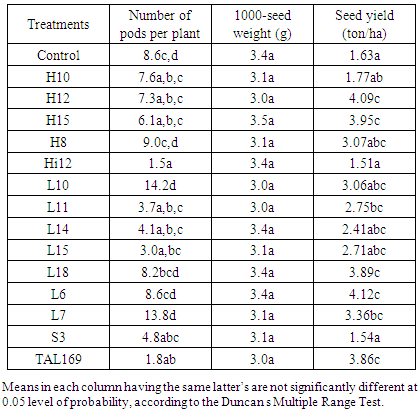
- Rhizobium strains significantly (p≤0.05) increased number of pods per plant as shown in Table 6. Inoculation with strains Treatments H10, H12, H8, Hi12, L10, L14, L6, L7 and S3 significantly increased number of pods per plant compared to other strains and control by 27%, 28%, 11%, 34%, 13%, 15%, 55% and 9% respectively. Comparable results were reported by Ibrahim et al, [20] in guar. Inoculation of guar with local and introduced strains had significant effect (p≤0.05) on number of pods in both sites. On the other site the inoculation of guar genotype with strains H8, L10, L14 and L7, significantly increased the number of pods by 5%, 66% and 61% respectively. These results were found to be on the same line with those obtained by Gomaa and Mohamed [3]. Rhizobium inoculation had no significant (p≤0.05) effect on 100-seed weight (g) of the guar (Table 6). While strain H15 increased 100-seed weight by 2.34%. These findings are in line with finding of Ibrahim et al [21] and Elsheikh et al, [22]. Inoculation of guar genotype with rhizobium strains significantly (p≤0.05) increased the final seed yield (ton ha-1). Whereas treatments H8, Hi12, L10, L11, L14, L15, L18, L6, L7, S3 andTAL169 increased the final yield by 9%, 5%, 34%, 6%, 49%, 53%, 6%, 15%, 47%, 40% and 18% respectively, compared to un-inoculated one (Table 5). These results supported the findings of Ibrahim et al [14] and Bhuiyan [15] in Chickpea.
4. Conclusions
- The results indicated that inoculation of guar with Bradyrhizobium (local and introduced strains) produced greater growth and seed yield in all inoculation treatments. So as a result inoculation of guar crop with Bradyrhizobium is recommended for similar conditions with this study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML