-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(6): 291-304
doi:10.5923/j.ijaf.20150506.01

Duration of Blossoming, Longevity of Argan Flower and Assessment of Pollen Fertility by Staining
Benlahbil S.1, Zahidi A.2, Bani-Aameur F.1, El Mousadik A.1
1Laboratory of Biotechnologies and Valorization of Natural Resources, Faculty of Sciences, Ibn Zohr University, Agadir, Morocco
2Polydisciplinary Faculty Taroudant, Laboratory of Biotechnologies and Valorization of Natural Resources, Agadir, Morocco
Correspondence to: Zahidi A., Polydisciplinary Faculty Taroudant, Laboratory of Biotechnologies and Valorization of Natural Resources, Agadir, Morocco.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

To determine time, flower opening and flower longevity in Argania spinosa; the number of flower buds (BF), flower bud with an emerging style (BFS) and open flower (FE) were counted in the field in three essays. Among observed genotypes in Ait Melloul site, BF will become a BFS in a period of 11 days.The degrees of opening of argan flower are conducted as follows: swelling of BFS and opening of the first petal in FE0, appearance of the first stamen in FE1, appearance of three stamens in FE2 and then the remaining stamens in FE3, the full opening of flower in EF4. The interval between FE0 and the full opening of the flower (FE4) will extend in two hours and 45 minutes. Once bloomed, FE remains opened for four days to reach FSCP phase (dry flower with a corolla). The longevity of argan flower was about 17 days depending on genotype. During the 4 days during which FE4 becomes FSCP, pollen grains are still present at the stamens. Evaluation of pollen fertility of the three floral stages BF, BFS and FE was performed by staining using two vital dyes acetic carmine and Alexander’s stain. Pollen grains are available at the anthers from BF stage; but are not mature enough to germinate until open flower (FE). High differences in pollen fertility were revealed for different geographical origin and genotype. Only 56% of pollen was viable in Argana site compared to Ait Melloul (73%). In argan forest, within-population variation in abiotic resource availability and in weather conditions was the rule rather than the exception. The opening of flowers and mature pollen are not synchronous among all the trees. So, flower phase FE is at its population maximum for twenty days in spring, contributing with fertile pollen and receptive stigmas to within-population gene flow. The long floral duration can function as a mechanism to avoid competition for pollinators and provide reproductive assurance in this species.
Keywords: Argania spinosa, Acetic carmine, Alexander’s stain, Flower longevity, Floral phase, Genotype, Pollen fertility
Cite this paper: Benlahbil S., Zahidi A., Bani-Aameur F., El Mousadik A., Duration of Blossoming, Longevity of Argan Flower and Assessment of Pollen Fertility by Staining, International Journal of Agriculture and Forestry, Vol. 5 No. 6, 2015, pp. 291-304. doi: 10.5923/j.ijaf.20150506.01.
Article Outline
1. Introduction
- Successful breeding depends on knowledge of floral biology of the species. Flower longevity defined as the length of time of an individual flower remains open in the field with fresh appearing perianth, stigma, and stamens was important in understanding pollination ecology as a dynamic process. It was also defined as the number of days elapsed between anthesis and early flower senescence, and was crucial for the pollination process (Singh et al., 1992; Scott et al., 1994; Vara Prasad et al., 2000). The longevity of flower depends on its morphology which is very variable depending on species; variety and genotype (Scott et al., 1994; Karle and Boyle, 1999). According to Eckhart et al. (1996), individuals with the ability to complete quickly the process of reproduction; are favored under natural conditions. This rapidity can only be achieved by a rapid development of floral organs thus inducing small flowers (Runions andGeber, 2000). Unpollinated flowers remain on the branches for a longer period (Scott et al., 1994; Karle and Boyle, 1999). Flower longevity, the period during which a flower is fully open and functional, varies greatly among the different plant species ranging from a few hours to weeks or even months (Primack, 1985; Van Doorn, 2003). The length of time a flower is functional may be an important determinant of male and female reproductive success (Evanhoe and Galloway, 2002; Rathcke, 2003; Itagaki and Sakai, 2006; Makrodimos et al., 2008). In argan tree, isolated or grouped flowers in glomerule (more than 14 units) are hermaphrodites (Perrot, 1907; Bani-Aameur et al., 1998). The transformation of flower unfolds in six phenological phases: flower bud (BF) (1 to 2 mm), flower bud with an emerging style (BFS) (1 to 2 mm), blooming flower (FE) (2 to 3.15 mm), dry flower with a corolla FSCP (2 to 3.15 mm) and finally dry flower with no corolla FSC (1 to 2 mm), then knotted fruit which begins at 2 mm (Bani-Aameur, 2000). In several species, the flowers bloom before or simultaneously to anthesis (Runions and Geber, 2000; Boyle, 2001; Anderson and Hill, 2002). In argan; the pistils are receptive to pollen released from the stamens at the FE stage (blossoming flower or open flower) (Benlahbil and Bani-Aameur, 2003). The study of pollen fertility is essential for quality assessment during the pollination, and therefore controlling the transmission of genes between plant generations. Pollen grains are sensitive to different biotic and abiotic factors during flowering. The fluctuation of these factors during the same day requires a rapid assessment of pollen fertility (Baez et al., 2002). The determination of an appropriate method is the key to a proper assessment of this fertility (Rodriguez-Riano and Dafni, 2000). Studies in several species have shown that the estimation of fertility by staining is positively correlated with number and quality of fruit from pollination (Widrlechner et al., 1983; Ortiz et al., 1999; Vara Prasad et al., 1999). For the study of pollen fertility, several vital dyes and technics are used as acetic carmine and Alexander’s stain (Brewbaker and Kwach, 1963; Widrlechner et al., 1983; Bajaj et al., 1992; Mulugeta et al., 1994; Rodriguez-Riano and Dafni, 2000; Song et al., 2001). Acetic carmine staining was positively correlated with in vivo pollen germination (Janssen and Hermssen, 1976; Vachun, 1981; Chang and Neuffer, 1989; Mamatha et al., 1993; Mercado et al., 1994; Fernández-Muñoz et al., 1995; Ascher and Peloquin, 1966, 1968; Volger et al., 1999; Cavalcante et al., 2000; Ramsey and Vaughton, 2000; Domínguez et al., 2002). The results obtained by Bani-Aameur (2002) following the study of pollen viability in argan using acetic carmine are promising. Using the Alexander’s stain in other species (Dumas, 1984; Mulugeta et al., 1994; Dag et al., 2000; Baez et al., 2002; Pline et al., 2002), and our preliminary observations encourages us to test the efficiency of this stain in argan for assessing viability of pollen. In natural populations of argan, despite considerable theoretical interest of flower longevity in pollination success and fruit formation, relatively little work has focused on relationships between opening of flower, the time during which the flower remain open, functional and able to receive pollen and pollen fertility. To fully understand this relation, we address several questions concerning the flower blossoming from BF to FE stage, longevity of each stage in the field. To determine pollen viability, we tested two vital dyes, Alexander’s stain and to carmine acetic, compared to in vitro germination, using fresh pollen collected in the field from each phase of argan flower and brought to the laboratory.
2. Material and Methods
2.1. Duration of Blossoming, Flower Longevity
2.1.1. Test 1: Transformation of BF, BFS and FE
- We conducted field work during March and April in the Agronomy and Veterinary Institute of Agadir southwest of Morocco whose ecological characteristics were cited in Bani-Aameur and Zahidi (2005). Three genotypes are taken randomly in the Horticultural Complex Ait Melloul. On each of the two shoots (STY) of each genotype; numbers of BF (flower bud), BFS (flower bud with an emerging style) and FE (open flower) from less to more blooming were recorded on five glomerule (a cymose clusters of up to 15 pentamerous flowers) from the basis (position 1) to the apex (position 5). Measurements were conducted between 8:00 and 16:00 every day for one week during March 19 to 25; period characterized by a maximum of flowers according to Bani-Aameur, 2000.
2.1.2. Test 2: Transformation of Inflated BFS to FE
- To determine the time, and flower opening steps, using a headband attachment magnifier, we selected experimental flowers in the same developmental stage as flower buds with an emerging style (BFS) (2 mm). Flowers that turned out to be FE were discarded from the experiment. Two BFS inflated, are taken at random on shoot over two years (STY) (fruiting shoot according to Zahidi, 1997; Zahidi et al., 2013), and are followed during one day in March in Ait Melloul site.
2.1.3. Test 3: Transformation of BF Until Senescence: Flower Longevity
- To examine if flower longevity differed among genotypes and localities, we measured the time from the flower bud (BF: 1mm, when perianth flower bud completely enclosed in protective green scales is visible) to wilting (when petals started to loose their color and started to drop off the flower) in each site. Thus, following information obtained from the previous two tests; a daily monitoring was conducted from April 14, of flower bud (BF) until their transformation to dried flower with persistent corolla FSCP. FSCP is characterized by corolla and androecium which become tarnished yellowish brown (Bani-Aameur, 2000). The transformation of two BF in each of the ten glomerule from position 1 (base) to position 10 (apex) is followed in six shoots (STY) in all three genotypes of test 2. All BF transformed into BFS and then to FE are counted each day at 8:00 morning. The flower is considered blossomed since the opening of the first petal. All FE are followed over time to determine their life until complete senescence.
2.2. Pollen Fertility
2.2.1. Comparison between Two Vital Dyes
- In full bloom, March 25 to 27, the flowering shoots (STY) sampled in thirty genotypes in each of three localities: Ait Melloul (AM), Argana (AR) and Ait Baha (AB) are immediately placed in refrigerator after harvest. Flower buds are removed and put into pill and then stored at -20°C. Pollen is collected by dissections performed under binocular magnifier by dissections (G x 20). Pollen grains harvested, are stained with two vital dyes, acetic carmine and Alexander’s stain, which has been used to distinguish between viable and nonviable pollen in many species. Acetic carmine can distinguish viable pollen grains; which are colored bright pink and are characterized by regular wall (Janssen and Hermssen 1976; Ascher and Peloquin, 1966, 1968; Volger et al., 1999; Ramsey and Vaughton, 2000). Pollen colorless and / or with malformations with a heterogeneous cytoplasm are considered as nonviable. Alexander’s stain contains malachite green, which stains cellulose in pollen walls and acid fuchsin, which stains the pollen protoplasm (Baez et al., 2002). Barrow (1983) reported that Alexander’s stain can distinguish pollen grains that aborted early in development (did not develop a protoplasm) from mature pollen grains; however, pollen aborted near maturity may also reduce determination capability and would not be detected. Thus, viable pollen grains are colored in purple, but nonviable grains were green (Dumas, 1984; Dag et al., 2000, Baez et al., 2002). Observations are made under microscope (G x 40). Percentage of viable pollen is estimated on two samples of 200 grains each.
2.2.2. Correlation between Staining and in Vitro Germination
- Flowers at BF, BFS and FE stages of 14 genotypes originated from Ait Melloul are harvested in March. These flowers are immediately dissected under binocular loupe (G x 20) to retrieve fresh pollen. Pollen fertility is estimated by:- Staining: pollen was stained by Alexander’s stain and observed as described in the previous test.- In vitro germination: germination and observation of germinated pollen are performed according to method described by Benlahbil and Bani-Aameur (2003).
2.3. Statistical Analyses
- For time of blooming of argan flower and floral longevity, we used an analysis of variance (ANOVA) with two (genotype and glomerule position); or four (genotype, glomerule position, day and hour) in hierarchical model to assess if there were differences among all factors (Dagnelie, 1984). For viable pollen, an analysis of variance with three factors (dye, locality and genotype); or two factors (genotype and floral stage) were performed for the number of viable pollen and number of germinated pollen in vitro. The least significant difference test (LSD α = 5%) of equality of means was used to compare differences between means (Steel and Torrie, 1960; Sokal and Rohlf, 1995). A correlation was used to compare percentages of viable pollen revealed by the two solutions; and the percentages of viable pollen by Alexander solution only and percentages of germinated pollen in vitro (Snedecor and Cochran, 1973; Bernstein et al., 1988). All statistical analyzes were performed Statitcf, Statistix software.
3. Results
3.1. Duration of Blossoming, Flower Longevity
3.1.1. Test 1: transformation of BF, BFS and FE
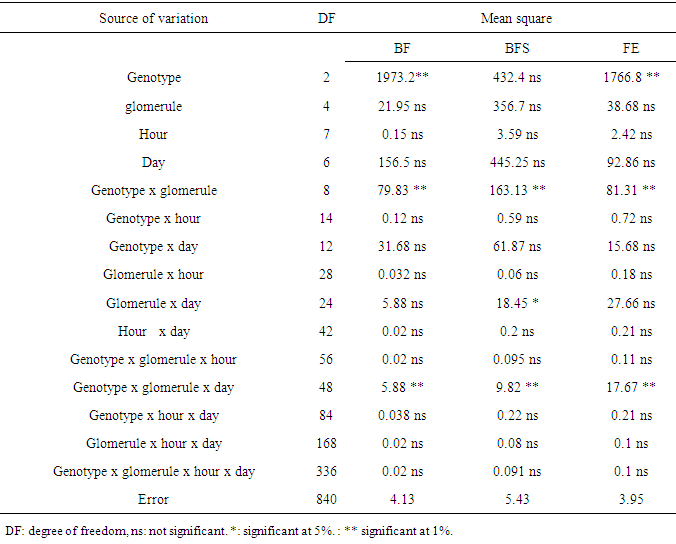
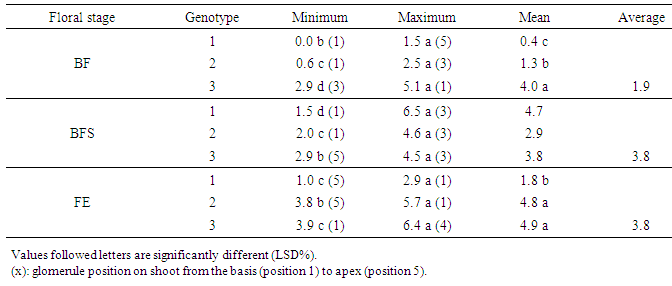
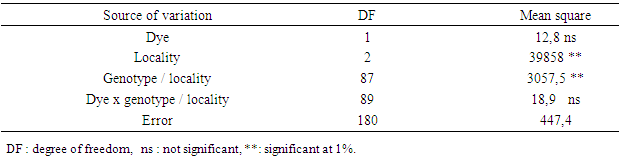
- The hour of day and day factors were not significant for the three floral stages (Table 1). Hour x day, hour x gomerule, glomerule x hour x day interactions were not significant for the three traits. From 1st to 7th day, number of BF (flower bud) decreased from 3 to 1 unity, from 6 to 2 for BFS (flower bud with an emerging style). Number of FE (blooming flower) oscillated from 2.5 in the 1st day to 4.6 the 7th day (Figure 1). Genotype x glomerule x day interaction was highly significant for the three stages. Genotype factor was significant for number of BF and FE; but not significant for number of BFS (Table 1). Depending on genotype, number of BF and FE varied respectively from 0.4 to 4, and 1.8 to 5 unities per glomerule (Table 2). Genotype x hour, genotype x day, glomerule position had no effect on three traits. Interaction genotype x position of glomerule was highly significant for all three phenological phases.
|
|
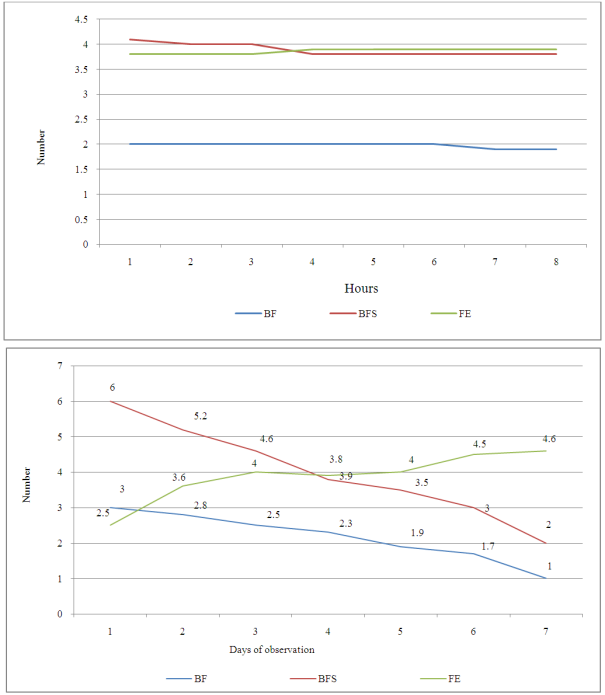 | Figure 1. Average number of floral bud (BF), floral bud with an emerging style (BFS) and blooming flower (FE) observed in each hour and day in three genotypes |
3.1.2. Test 2: Transformation of Inflated BFS to FE
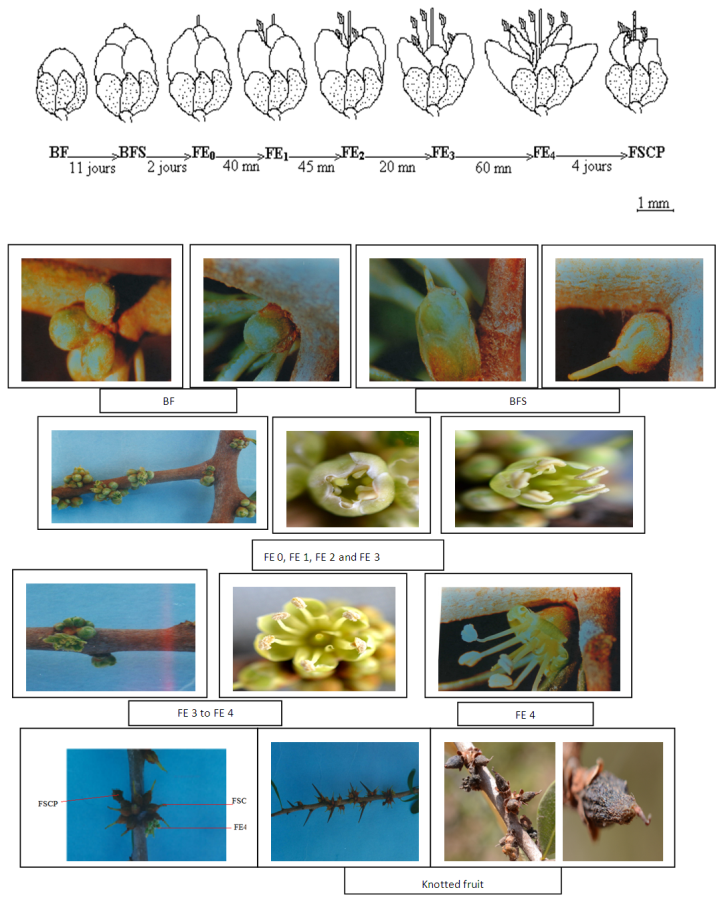
- Flower bud with an emerging style inflated is transformed to (FE0). FE0 is characterized by the opening of one of its petals, with appearance of the first stamen after 40 minutes. This is the first degree of opening of the open flower (FE1) (Figure 2). About 45 minutes later, two other stamens, appear simultaneously on opposite side, showing a second level of blossoming (FE2). The opening continues until exit of the last two stamens. This third degree of openness (FE3) is obtained 20 minutes after FE2. The blossoming of the corolla would be complete after one hour (EF4). This last stage (FE4) remains on shoot until the next day. The approximate life span of the four phases of this floral stage (FE) was respectively: 45 min for FE1, 20 minutes for FE2, one hour for FE3, and more than 24 hours for FE4.
3.1.3. Test 3: Transformation of BF until Senescence: Flower Longevity
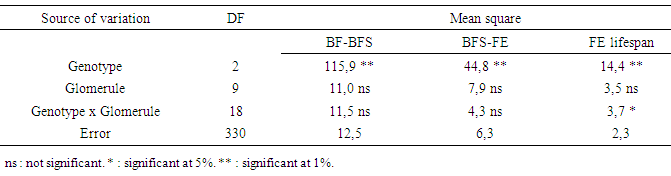
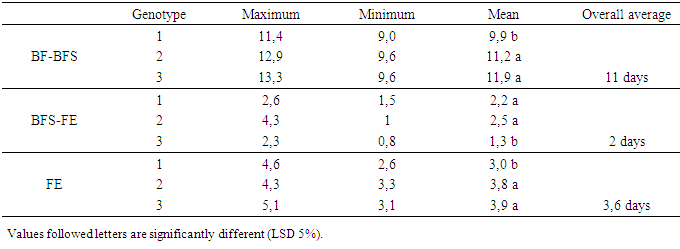
- Genotype factor was highly significant for number of days necessary for transformation of BF to BFS; BFS to FE and FE lifespan (Table 3). The glomerule factor was not significant for the three studied characters. Genotype x glomerule interaction was significant only for FE lifespan. Depending on genotype, transformation of BF to BFS occurs between 10 and 12 days, with an average duration of 11 days (Table 4). Floral stage BFS evolves to FE during a period between one and three days depending on genotype. The lifetime flower once bloomed (FE) ranges from three to five days depending on genotype.
|
|
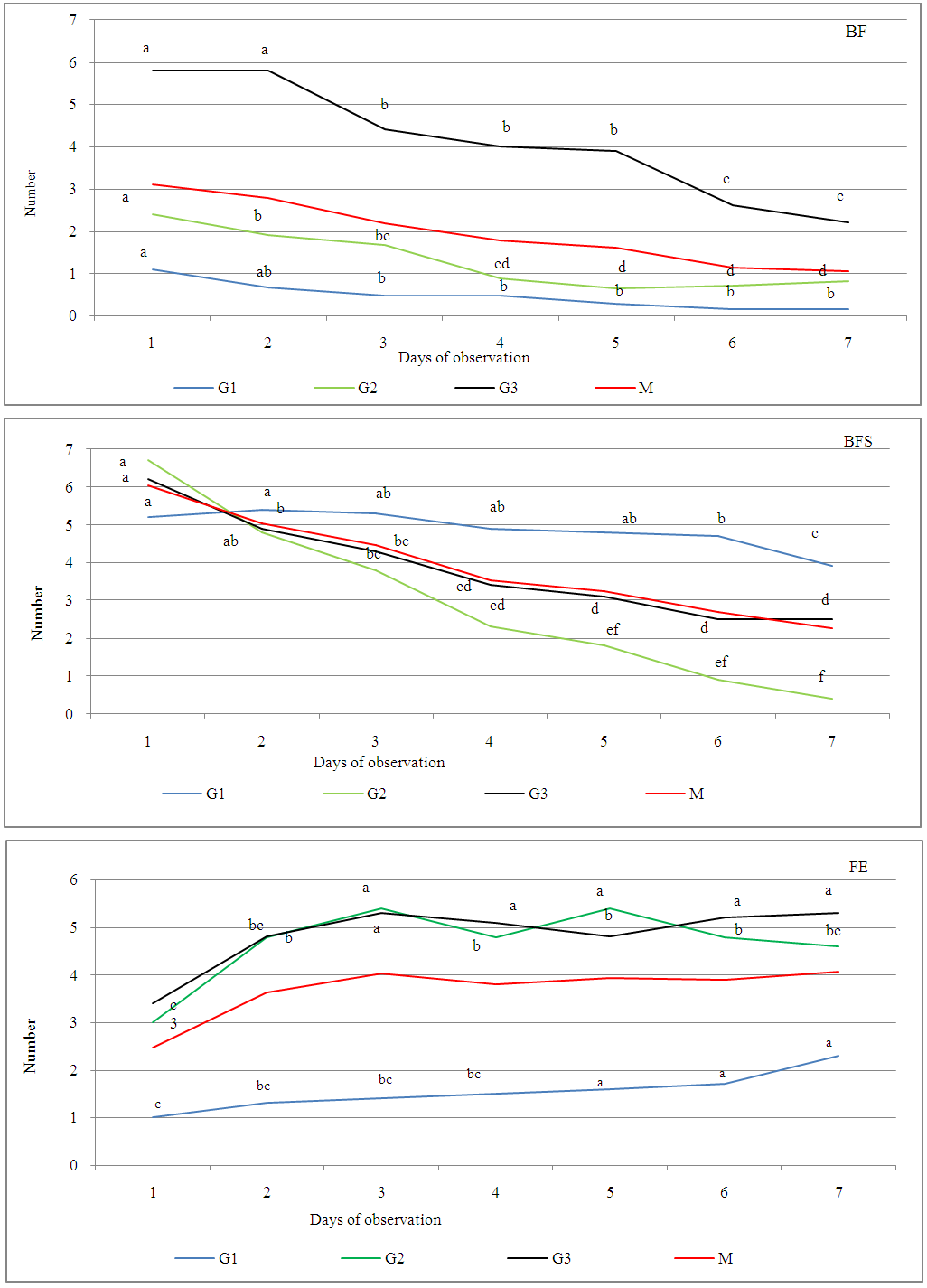 | Figure 3. Number of flower buds (BF), flower bud with an emerging style (BFS) and blooming flower (FE) per glomerule observed in one week in three genotypes |
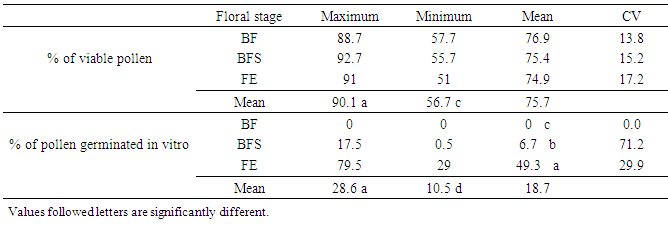
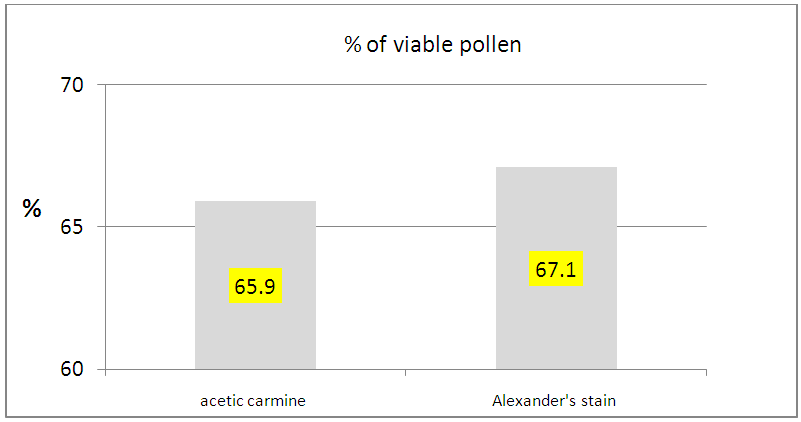 | Figure 4. Percentage of viable pollen stained with acetic carmine and Alexander’s stain |
3.2. Pollen Fertility
3.2.1. Comparison between the Two Vital Dyes
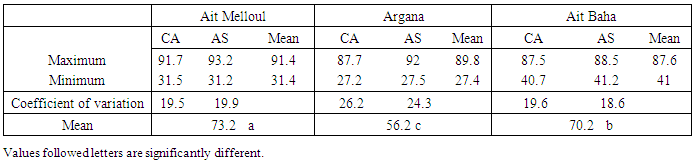
- Dye factor was not significant for percentage of viable pollen (Table 5). Both stain showed identical percentages of viable pollen, 65.9% (acetic carmine) and 67.1% (Alexander’s stain) (Figure 4). However, Alexander’s stain (Figure 5a); allows faster observation of viable pollen compared to the acetic carmine dye (Figure 5b). Locality factor was highly significant for the percentage of viable pollen (Table 5). The maximum percentage is observed in Ait Melloul (73%), and in Ait Baha (70%) (Table 6). Argana site shows minimum percentage of viable pollen (56%). Genotype factor was highly significant for percentage of viable pollen (Table 5). Dye x genotype interaction in each site was not significant. Genotypic variation is detected by the two dyes, but average percentage of viable pollen varied in large proportions by genotype.
|
|
3.2.2. Correlation between Staining and in Vitro Germination
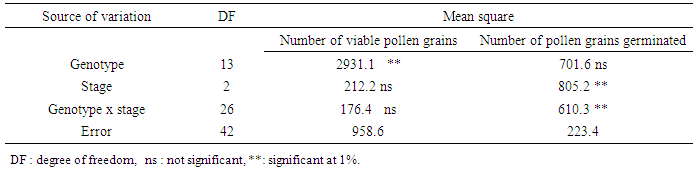
- Genotype factor was highly significant for percentage of viable pollen stained with Alexander’s stain. Floral stage and genotype x floral stage interaction were not significant (Table 7). Genotype factor was not significant for percentage in vitro germinated pollen but floral stage and genotype x floral stage interaction were highly significant. The average percentage of viable pollen was four times higher than average percentage of pollen germinated in vitro (Table 8, Figure 5c). Percentage of viable pollen varied in large proportions by genotype. Alexander’s stain showed no difference between the pollen issued from the three floral stages. Pollen of floral bud stage (BF) does not germinate in vitro, while percentage of germinated pollen obtained from BFS (6.7%) was seven times less than pollen from FE stage (49.3%). The coefficient of variation of this percentage between genotypes was important in two stages and especially in BFS.
|
|
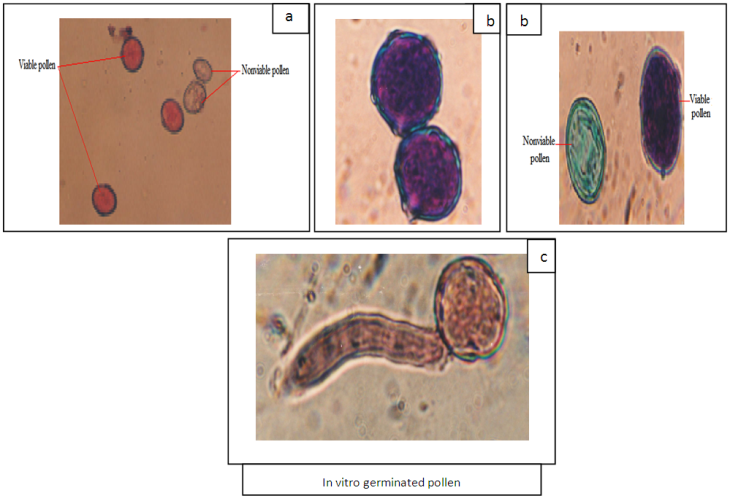 | Figure 5. Pollen grains of argan flower stained with acetic carmine (a) (scale = 10 µm), and Alexander’s stain (b) (scale = 15 µm) and in vitro germinated pollen (c) (scale = 10 µm) |
4. Discussion
- The hour and day factors were not significant for the three stages floral, showed that transformation of phenological stages of argan flower does not occur at the scale of hour, or one day or even for a week. Fluctuations in number of blossoming flowers (FE) from the 3rd to 7th day can be explained by physiological drop in FE already formed or their transformation to knotted fruits on one hand; or the emergence of new FE after changing of BFS obtained from BF on the other hand. The time required for conversion of BF into FE is revealed independent of glomerule position on shoot. This finding has also been observed in other species of the family Onagraceae (Runions and Gebee, 2000). According to Louali (1994), counting number of flowers every twenty days showed a significant difference between each observation. Thus, the transformation of BF to FE will take place for a period strictly between seven and twenty days. Among the genotypes observed in this study in Ait Melloul, valley site characterized by mild temperatures; floral stage BF will become a BFS in a period of 11 days on average. BFS is characterized by the emergency of the stigma of the bud at the early stages and of part of the style, which elongates out of the flower bud still enclosed in protective green scales. Therefore the stigma is available to receive foreign pollen from other flower (Bani-Aameur, 2003). BFS will become FE0 in two days. The degrees of opening of argan flower are conducted as follows: swelling of BFS and opening of the first petal in FE0, appearance of the first stamen in FE1, appearance of three stamens in FE2 and then the remaining stamens in FE3, the full opening of flower in EF4. The interval between opening of the first petal (FE0) and the full opening of the flower to reach FE4 will extend in two hours and 45 minutes. During the 4 days during which FE4 becomes FSCP, pollen grains are still present at the stamens (Zahidi et al., unpublished data). In some species, as Oenothera biennis, flower opening was often rapid and takes less than 20 min and in Hedera helix it occurs in about 5 min (cited in Van Doorn and Van Meeteren, 2003). In other species, the time between the opening of the first petal and the full opening of flower is extended between 2 and 12 hours (Rigby and Dana, 1972) and can last up to four days (Petanidou et al., 1995; Ownbey and Maloof, 2000; Anderson and Hill, 2002). In addition, Runions and Geber (2000) reported that development of flower bud (1-2mm) to an open flower lasts between 23 and 34 days depending on variety and genotype. In argan tree, the flower bud becomes an opened flower in a variable period depending on genotype, and it occurred during an average period of 13 days. Once bloomed, the FE remains opened for four days to reach the stage FSCP (dry flower with a corolla). Thus, this tree of Sapotaceae family was among the species whose flowers open during the day, and remained open during four days. As reported in literature, floral opening in many species that bloom in spring, occurs in the morning, correlated with an increase in temperature and light intensity, and with a decrease in ambient humidity (Van Door and Van Meeteren, 2003). During observation period (March), the maximum temperature was between 23°C and 33°C. The minimum temperature varies from 11°C and 15°C. The minimum and maximum relative humidity varies between 35% respectively 74% and 80% to 95%. Those conditions will be probably favorable for opening of argan flowers. In a subsequent paper, variation in environmental factors (e.g. rainfall, temperature) contributes directly or indirectly to generate heterogeneity on flowering phenology in the three studied populations (Zahidi et al., 2015). The full bloom period was observed in winter – spring. It varied from 18 days in winter in Argana, to 42 days in spring in Ait Baha population. The minimum flowering duration per tree was about 60 days in Ait Baha. Two trees in Argana (11 and 20) have flowers in only 20 days. Flowering in autumn and summer was less important and interested a limited number of trees. Sprawl of flowering over a long period represents an additional time which would be beneficial to increase rates of flower visitation for each pollinator, in obtaining pollination and ovules fertilization; although the success of pollination was certainly also affected by the speed with which each pollinator visits flowers (Vazquez et al., 2005). Since, the time of flowering and of flower opening marks the onset of a period in which pollinators will be attracted, leading to pollen removal in male and bisexual flowers and to pollination, fertilization and seed set in female and bisexual flowers. Floral longevity was assumed to reflect a balance between the benefit of increased pollination success and the cost of flower maintenance. It is conceivable that such variation in flower longevity may also occur among plants in different sites of contrasting pollinator visitation rates within a population (Primack, 1985; Bingham and Orthner, 1998; Fabbro and Korner, 2004). Primack (1985) observed that selfing species generally have shorter-lived flowers than outcrossing species of the same genus or family. He theorized that selfing species may benefit from pollinating quickly and moving on to fruit development, whereas an outcrossing species gains fitness by allowing flowers to remain open longer to increase the probability of visitation. The longevity of argan flower was about 17 days on average in the observed genotypes in Ait Meloul. But, length of time in which flower remains open in the field with a fresh-appearing perianth, stigma,and stamens exceeds five days. In some species, the longevity of flower does not exceed 9 hours (Ashworth and Galetto, 2001) while it oscillates between 4 and 12 days in several species (Scott et al., 1994; Karle and Boyle, 1999; Anderson et Hill, 2002). Flowers of Kalmia latifolia (Ericaceae), mountain laurel, have a long duration and can remain viable up to 21 days if unpollinated (Rathcke, 2003). Shrubs that flowered in spring had longer floral longevities of 6 to 9 days and a mean of 7.2 days, whereas shrubs that flowered in summer had floral longevities of 3 to 4 days and a mean of 3.4 d. The mean floral longevities were significantly different for summer- and spring-flowering shrubs. The floral longevity of K. latifolia was not significantly different from the mean floral longevity of spring-flowering shrubs, but the maximum floral longevity of Kalmia latifolia (Ericaceae) (21 days) was much longer than that found for any other shrub species in this community (Rathcke, 2003). Because stigma was able to receive pollen from BFS stage, pollen longevity defined as the time elapsed between his release from anthers and pollination (Camefort and Boue, 1985) was more than 6 days on average in Argan. However, pollen maturity and stigmatic receptivity in the argan tree was in their maximum at the FE stage (Benlahbil and Bani-Aameur, 2003). At this stage, the five anthers are dehiscent extrorsely by means of longitudinal slits making pollen available for dispersal. Indeed, among all female parents, number of pollen grains per stigma germinated after self-pollination was very low in comparison with the allo-pollinated flowers. As for distance traveled, pollen tube reaches 100% of the style in 22% in self-pollinated flowers on average. In no case, pollen tube reaches the ovary in self-pollinated flowers in two female parents. Thus, this pollen is functional as fertile male parent for four days which correspond to the open flower (FE) lifetime. After 4 days, FE is transformed to FSCP with corolla and androecium which become tarnished yellowish brown. This phase marks the limit to pollen dispersal. The style is still erect but we notice some color change toward the brown. This phase may also set the limit for stigma receptivity. According to Petanidou et al. (1995), in others species, four days are considered as a long life for the blossoming flower. In argan, FE lifetime (four days) appears to be favorable for a successful pollination allowing insects to perform multiple visits (Benlahbil and Bani-Aameur, 2003). If fertilization has not occurred, the FE loses its corolla and fall. For the realization of controlled crosses, flowers emasculation must be made at BF stage but pollination occurs only 13 days after emasculation.The time required for the passage from a phenological phase to another as well as flower longevity were variable depending on genotype. This time when flower remains open and functional could reduce or enhance the spatial distribution of pollen. Genotypic difference was observed in several other species (Deneke et al., 1990; Wu et al., 1991; Evensen et al., 1992; Scott et al., 1994; Karle and Boyle, 1999). According to Scott et al. (1994), the selection of genotypes with high flower longevity promotes pollination, since it conserves a high level of out crossing, and the effectiveness of the overall floral display in attracting pollinators when they are scarce. It is further assumed that floral longevity is heritable and can be optimized by natural selection in response to the pollination environment (Schoen and Ashman, 1995). This means that in environments with abundant, predictable pollinators plants would show shorter floral durations than in environments where pollinator activity is low.For pollen viability, two vital dyes were compared to determine their potential to estimate pollen fertility. Both Alexander’s stain and acetic carmine used for assessing viability showed identical results. As a result of the present study we recommend using in case of a large sample, Alexander’s stain, as a method for rapidly assessing pollen viability. According to Pline et al., (2002), Alexander’s stain gave the highest estimates of pollen viability (82-99%) compared to other methods. Contrary to results obtained by Mulugeta et al. (1994), pollen of argan flower, colored by Alexander’s stain will not fade even after several hours of staining.Pollen grains are available at the anthers from the BF stage; but are not mature enough to germinate until the open flower stage (FE). According to Song et al. (2001) assessment of fertility by in vitro pollen germination requires fresh and mature pollen. Thus, in argan tree, to estimate pollen fertility by staining or by in vitro germination; we must use pollen collected on FE and not from BF or BFS stages. Indeed, Alexander’s stain did not differentiate between pollen able to germinate and immature pollen. In others species, difference between mean percentage of viable pollen and average percentage of germinated pollen was greater than 50% (Adaniya, 2001a, b; Baez et al., 2002). These results are considered consistent according to Mulugeta et al. (1994). In argan tree, difference between average percentage of viable pollen stained with Alexander’stain and average percentage of germinated pollen was about 26% only. This small difference showed the good correlation between the two methods for estimating pollen viability. Difference in pollen fertility according to genotypes was detected in several species (Young, 1992; Parton et al., 2001). According to Ortiz et al. (1999), the variation between individuals detected by pollen staining is confirmed by molecular studies in several species. In our study, the difference between genotypes for pollen percentage viable and germinated pollen is clearly observed in argan tree. Difference between genotypes in pollen viability collected at BFS stage may suspect precocity in pollen maturity in some genotypes compared to others. These results will be useful for differential selection of genetic material in this tree in artificial pollination and breeding experiments. Pollen viability was highly dependent on environmental conditions that occur during its formation. Thus pollen grains are very sensitive to thermal variations at this stage (Schlichting, 1986; Bajaj et al., 1992; Petolino et al., 1992; Young, 1992; Mascarenhas and Crone, 1996; Mckee and Richards, 1998; Vara Prasad et al., 1999). The low percentage of viable pollen observed in Argana site (56.2%) compared to the two localities Ait Melloul (73.2%) and Ait Baha (70.2%) could be related to high temperatures recorded in this site of the high Atlas (Argana) during spring which coincides with period of pollen collection. The maximum average temperatures above 34°C caused a significant drop in flowers. Flowers that have supported and resisted at these high temperatures contain a fairly low percentage of viable pollen. In contrast, the average maximum temperature in Ait Mellou did not exceed 25.4°C in spring.Primack (1985) reported that selfing species may benefit from pollinating quickly and moving on to fruit development, whereas an outcrossing species gains fitness by allowing flowers to remain open longer to increase the probability of visitation. In addition, in outcrossing plants, flowering patterns determine the outcome of selection by influencing the amount of outcrossing (Murawski and Hamrick, 1991). In argan forest, within-population spatial variation in abiotic resource availability and in weather conditions was the rule rather than the exception. The opening of flowers and mature pollen are not synchronous among all the trees. So, flower phase FE is at its population maximum for twenty days in spring (Bani-Aameur, 2002), contributing with fertile pollen and receptive stigmas to within-population gene flow. Further researches are therefore needed to explore how such spatial variation in the availability of pollinators or abiotic conditions affects levels of pollen limitation on seed production among plants within a population.
5. Conclusions
- Among the genotypes observed in this study in Ait Melloul site, development of flower bud to an open flower lasts 13 days. Once bloomed, length of time in which flower remains open in the field with a fresh-appearing perianth, stigma,and stamens exceeds four days to reach the stage FSCP (dry flower with a corolla).Thus, the longevity of the flower argan was 17 days on average. In argan, FE lifetime (four days) appears to be favorable for a successful pollination allowing insects to perform multiple visits. The selection of long-life flower genotypes will be favorable for the success of controlled crosses. This study showed that evaluation of pollen fertility was performed by staining using two vital dyes acetic carmine and Alexander’s stain. The color difference between viable and nonviable pollen stained with Alexander’s stain was better and thus allows easier estimation of pollen fertility. But, this dye cannot distinguish between pollen able to germinate (mature pollen) and the unable pollen (immature pollen). High differences in pollen fertility were revealed for different geographical origin and genotype. The spread of flowering in argan over a period more than 20 days between winter and spring with remarkable differences between genotypes and geographic origins and with long floral duration can function as a mechanism to avoid competition for pollinators and provide reproductive assurance in this species
ACKNOWLEDGEMENTS
- We gratefully acknowledge anonymous reviewers and office journal which provided helpful comments that greatly improved the manuscript. We thank the Morocco-Germany Co-operative Project ‘Conservation Project and Development the argan forest’ (PCDA-GTZ) and the project Pars-Agro 128 of the Morocan Ministery of Scientific Research for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML