-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(4): 249-266
doi:10.5923/j.ijaf.20150504.06
Seasonal Variability in Flowering Phenology in Three Natural Populations of Argania spinosa (L.) Skeels
Benlahbil S. 1, Zahidi A. 2, Bani-Aameur F. 1, El Mousadik A. 1
1Faculty of Sciences, Ibn Zohr University, Laboratory of Biotechnologies and Valorization of Naturals Resources
2Polydisciplinary Faculty Taroudant, Taroudant, Laboratory of Biotechnologies and Valorization of Naturals Resources
Correspondence to: Zahidi A. , Polydisciplinary Faculty Taroudant, Taroudant, Laboratory of Biotechnologies and Valorization of Naturals Resources.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
An experiment was conducted for three consecutive years in three natural populations to investigate the effect of seasonal climate, geographical origin and genotype on flowering phenology in argan. Results revealed that water scarcity especially in dry and hot year induced a clear reduction in flowering and fruiting lengths to 38 and 147 days. But during the 3rd year characterized as wet following a very humid campaign, flowering duration and fruiting length were extended to 110 days and 300 days. The flowering peak in the studied areas occurred during March-April. In Ait Baha during dry year, flowering period was shifted in some individuals between June-August. Under such circumstances, extended flowering seems to be a strategy that minimizes reproductive failure. Ait Baha site where the effects of climatic year were perceived will be considered as a medium for selection of resistant genotypes to drought. There were striking differences among genotypes (tree / locality) in both flowering and fruiting durations. Some individuals were able to produce flowers even in very dry year and can conserve the formed fruits until 327 days. The flowering-fruiting cycle cover a period of 9 to 16 months depending on trees. For installation of orchards, improvement in argan requires selection of genotypes with a smaller number of days from bloom to harvest in order to ensure better fruit production under climatic variation of arid environments.
Keywords: Flowering duration, Fruiting length, Genotype, Seasonal variation, Arid environments
Cite this paper: Benlahbil S. , Zahidi A. , Bani-Aameur F. , El Mousadik A. , Seasonal Variability in Flowering Phenology in Three Natural Populations of Argania spinosa (L.) Skeels, International Journal of Agriculture and Forestry, Vol. 5 No. 4, 2015, pp. 249-266. doi: 10.5923/j.ijaf.20150504.06.
Article Outline
1. Introduction
- The study of phenological aspects of plants subject to different seasonal changes involves observation, recording and interpretation of the timing of their life history events. The phenology of leafing, flowering and fruit production in a range of species and communities were observed. The selective forces that influence the timing of these events are discussed as phenological patterns of each phase (timing, frequency, duration, degree of synchrony, etc). They are probably the result of compromise between a variety of selective pressures, such as seasonal changes, resource availability, and the presence of pollinators, predators and seed dispersers (Johnson, 1993; Mckitrick, 1993; Fenner, 1998; Medeiros et al., 2007; Sekhwela and Yates, 2007; Milla et al., 2010; Bartomeus et al., 2011; Soudani et al., 2012). Flowering phenology can evolve rapidly, as evidenced by the many examples of phenological divergence between plant populations within the same species, each adapted to their local conditions. Variation in environmental factors might also contribute to phenological variation in populations, but these phenotypic plastic responses themselves might also be genetically modulated to some extent (Quinn and Wetherington, 2002). The period and flowering duration are dependent on the species (Ng and Corlett, 2000; Elzinga et al., 2007). The change of flowering period over years has been the subject of several studies (Petanidou et al., 1995; Petanidou et al., 2014). Optimal time for flowering is defined as the period of balance between the intrinsic and extrinsic factors such as the availability of pollinators and climate conditions (Waser, 1979; Johnson, 1993; Eckert and Allen, 1997). As for the fruiting, it is closely linked to flowers production (Vara Prasad et al., 2000). The success of pollination depends on the balance between the successive periods of flowering and seasonal activity of pollinators. Early flowering and late flowering at the end of the season are unfavorable for successful pollination (Kudo, 1993). In argan early flowering trees, (precocious trees) the flowering onset occurred in October-December (Ferradous et al., 1996; Bani-Aameur et al., 1998). Late flowering trees (belated trees) start flowering in February-March; but the intermediate trees bloom along the humid period of the year. Result of flowering was fruit with varying productivity as well in early or late-trees. Other trees produce both types of fruits in the same fruiting year. Fruits of the precocious trees begin to drop in late April of the following year. The fruit abscission begins in August in belated trees. It extends between the spring and summer of the following year in the intermediate argan. The cycle of flowering and fruiting extends over two years in argan (Bani-Aameur et al., 1998). The number of glomeruli and the number of flowers per glomerule depends on rainfall and temperatures recorded during the flowering year (Bani-Aameur, 2000; 2002a). Prolonged drought during the flowering season is manifested by a significant reduction of the fruiting branches and number of fruits on twigs during the fruit ripening season. Contribution in the phenotypic variance of the climatic season and tree x environment interaction were very significant for studied characters (18.5 and 52.9%) (Zahidi et al., 2013a). This behavior towards climatic conditions varies also depending on tree genotype. Branching and shoot growth (shoot more than two years and fruiting branch) as well as flowering and fruiting depend on rainfall between December and late March. Precipitations after April did not have direct effects on the flowering in the same year (Zahidi, 1997; Zahidi et al., 2013a). Fruits productivity varied between 0.1 Kg (65.9 fruits) in dry season in Ait Baha to 94.4 kg (29298.2 fruits) during the wet season in Argana. For other fruit components, values varied from 0.13 kg for almonds to 7.9 kg for kernels. Oil content in almonds varied from 39.2% to 44.1%, but estimated annual yield of argan oil varied from 0.1 kg to 2.6 kg / tree (Zahidi et al., 2014a). The multiple uses of argan, especially the interest of oil combined with resistance to drought, make it a good candidate for domestication as a fruit tree and last defense against desertification in south Morocco (Bani-Aameur and Benlahbil, 2004; Ait Aabd et al., 2011). The fluctuation of periods of flowering and fruiting as well as variation in number of trees bearing flowers and fruits depending on climatic conditions are still unknown. Our objective was to explore whether the seasonal changes (e.g. temperature and precipitation), geographical origin affect flowering phenology and fruiting duration in argan in order to identify the genotypes with a good potential for cultivation or for exploitation in breeding programs.
2. Materials and Methods
2.1. Study Site
- The study was carried out in a semi-arid and arid environment and concerned three natural populations followed for over a ten years in several morphological characters; Ait Melloul, Argana and Ait Baha (Bani-Aameur and Zahidi, 2005). Rainfalls during the three consecutive years of study are often scarce and variable; taking place mainly during the cold period while summer was dry. The 1st was dry and hot, but both years (2nd and 3rd) were wet, but differed in the distribution of seasonal precipitation. The years had relatively humid springs, while the summer drought extended well into fall (Figure 1a, b).
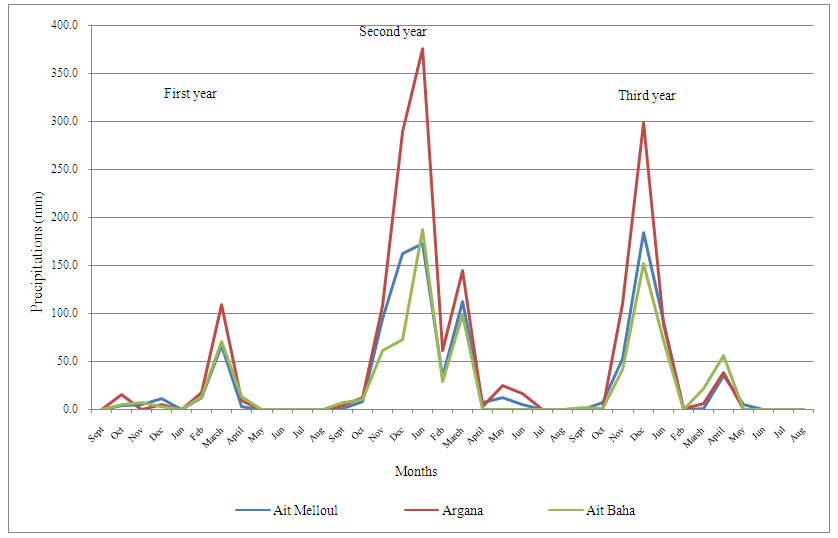 | Figure 1a. Data on total precipitation per month for the three study years in the three localities |
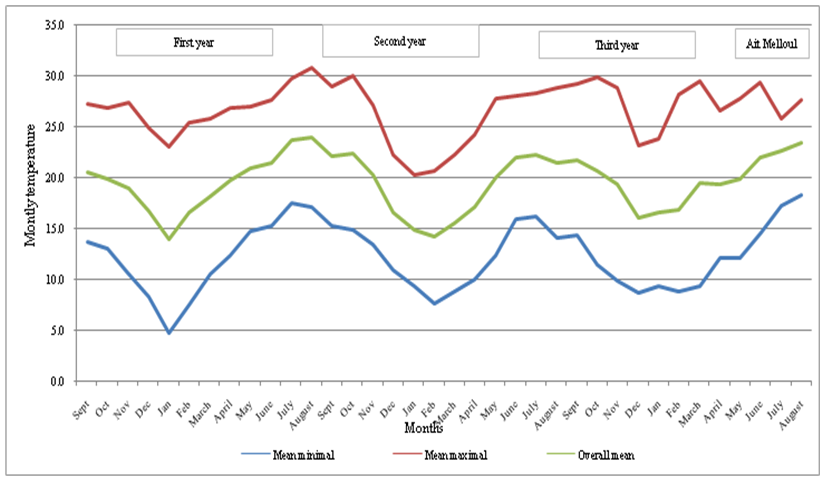 | Figure 1b. Monthly temperature data (overall mean, mean maximal and mean minimal) of the study site Ait Melloul during the three years |
2.2. Data Collection
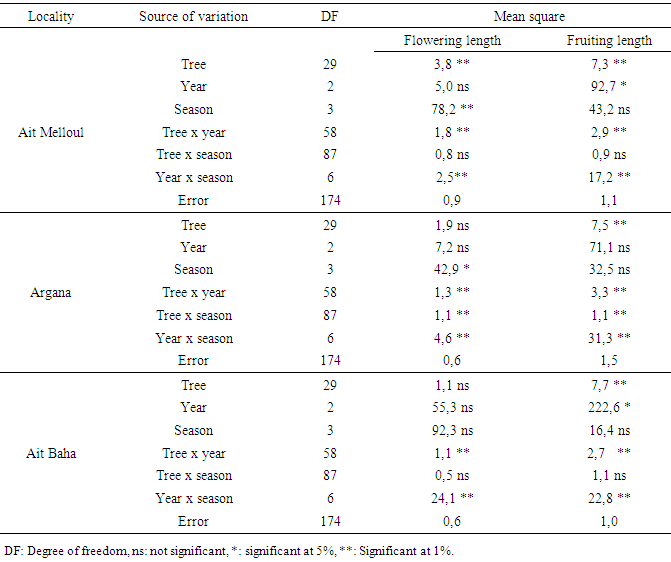
- To explore whether the seasonal changes of temperatures and precipitations affects the flowering phenology and the fruiting phenology in argan; thirty trees were randomly selected from each site which were the subject of several previous studies for morphological characters of fruit, branching, flower, yield and pollen (Ferradous et al., 1996; Zahidi, 1997; Bani-Aameur and Ferradous, 2001; Bani-Aameur, 2002b; Zahidi et al., 2013a). Trees were selected and tagged before the onset of flowering. Plants were visited every twenty days between September and August during the three consecutive years. At each census, we noted the presence or absence of flowers in tree at different stages (Bani-Aameur, 2000). We noted also the presence or absence of fruit (young fruits from flowering of the year) or fruits during ripening derived from flowering of the previous year. We consider flowering length (flowering duration) per tree as the number of days in which flowers are present on the tree during each season (autumn, winter, spring and summer), and each year. The fruiting length (fruiting duration) per tree was defined as the number of days in which fruits are present on plant in every season and every year. Number 1 was given for the presence of flower or fruit, the number 0 indicated the absence of flower or fruit. We deduced, the number of trees bearing flowers or fruits per season, per year in each site.
2.3. Data Analysis
- An analysis of variance (ANOVA) in hierarchical model with three factors (locality, year and tree) or four factors (locality, year, season and tree) was adopted (Dagneli, 1984). Tree is hierarchical to locality factor because trees were not repeated in each site. Factors year, season and locality were crossed. Least significant difference method (LSD α = 5 %) was used for mean separation; where significant differences were obtained (Steel and Torrie, 1960; Montgomery, 1984; Sokal and Rolf, 1995). Genotypes were grouped using UPGMA (unweighted pair-group method arithmetic average). Dendrograms were performed on averages of periods of flowering or fruiting per tree. Statistic computations were performed using Statistix, Statitcf, NTCYS software (Version1.5, Rohlf, 1988).
3. Results
3.1. Variability of Flowering and Fruiting Lengths
3.1.1. According to the Year and Locality
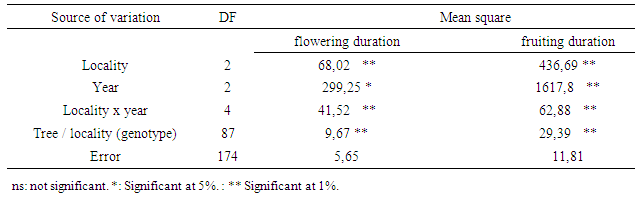
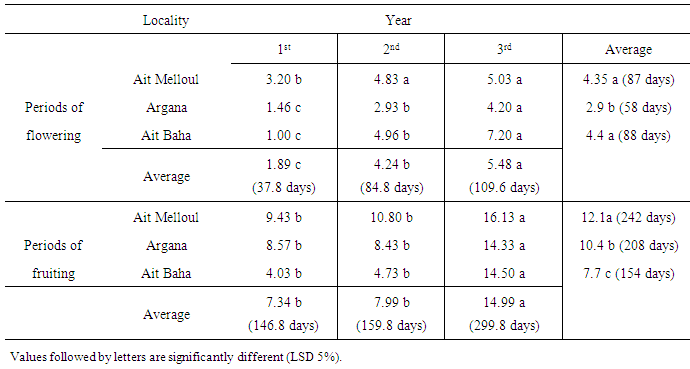
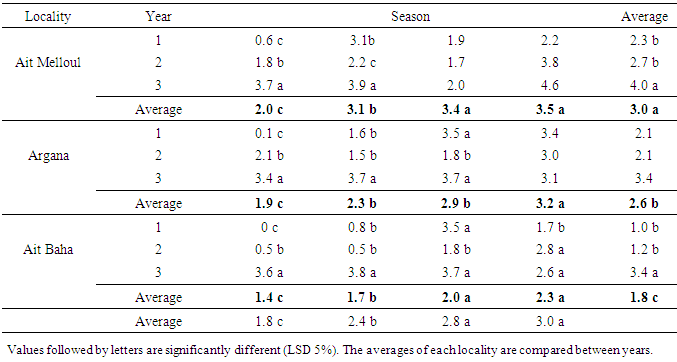
- Locality, year and locality x year interaction were significant for flowering length and fruiting length (Table 1). During the three years, the average flowering duration was about 88 days in Ait Baha site; 87 days in Ait Melloul and only 58 days in Argana (Table 2). The fruiting length was also variable between the three geographical sites during the three years. The average fruiting duration was respectively 242 days in Ait Melloul, 208 days in Argana and only 154 days in Ait Baha. In all three sites; during the driest year (1st) following a very dry and hot campaign; the flowering durations (38 days) and those of fruiting (147 days) were shorter (Table 2). During the 3rd campaign characterized as humid following a very wet year (2nd), length flowering (110 days) and fruiting duration (300 days) were longer. Intermediate values were recorded during the second year, respectively 85 and160 days.
|
|
3.1.2. According to Season
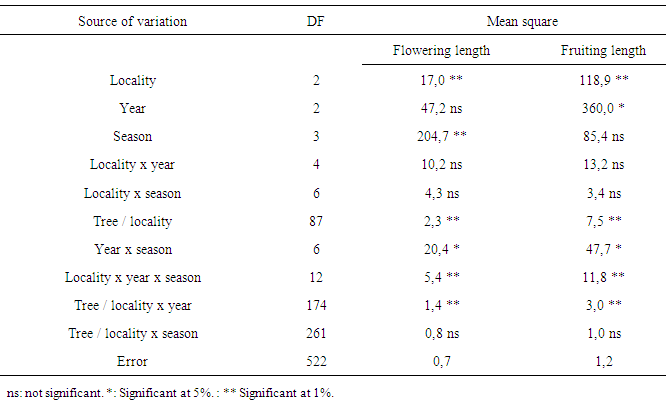
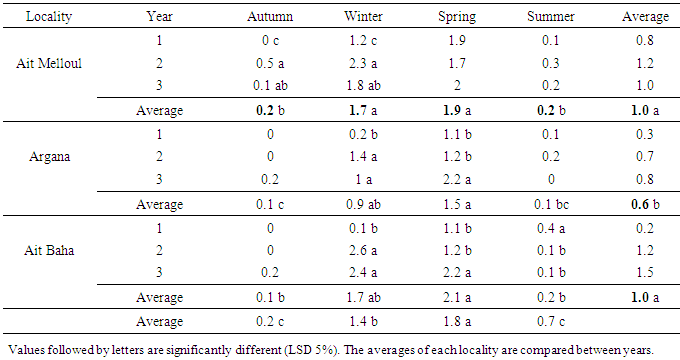
- Locality had affected significantly the flowering length and fruiting duration (Table 3). The flowering duration in each season was statistically identical in Ait Melloul and Ait Baha populations while they were shorter in Argana locality (Table 4). The fruiting length was longer in Ait Melloul than those in Argana and in Ait Baha (Table 5). We detected no effect for year, locality x year, locality x season interactions for both characters. High seasonal variability for flowering length; but no effect of season was observed for fruiting duration (Table 3). Year x season and locality x year x season interactions were significant for both characters. Flowering intensity across the year was extremely variable, with a clear flowering peak observed in winter - spring, almost low flowering in summer and autumn. Thus, flowering duration was longer in spring (38 days) and winter (34 days) than during autumn and summer (4 days) in Ait Melloul population during the three years. In Argana, flowering duration was shorter in autumn and summer (2 days) than in spring (30 days) and winter (18 days) (Table 4). At Ait Baha, the flowering length was shorter in summer (0.2 x 20 days) and autumn (0.1 x 20 days) than in spring (42 days) and winter (34 days).
|
|
|
3.1.3. According to Genotype
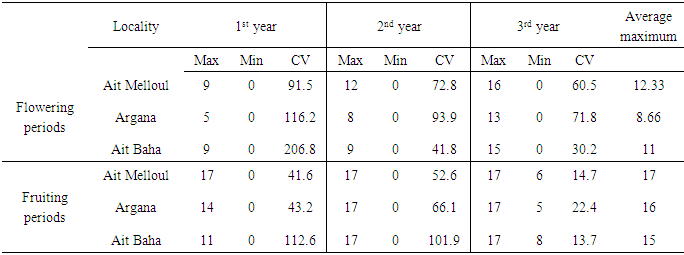
- We found a significant effect of tree genotype (tree / locality) for both durations of flowering and fruiting (Table 1). During the three years, the maximum flowering length per tree was observed in Ait Melloul (320 days), about 20 days longer than the maximum recorded in Ait Baha and about 60 days than that observed in Argana site (Table 6). The minimum flowering duration per tree was about 60 days in Ait Baha. Two trees in Argana (11 and 20) have flowers in only 20 days. Depending on the genotype, the average fruiting period varies from 100 to 327 days in Ait Melloul, 107 and 320 days in Argana and oscillates between 27 and 294 days in Ait Baha. The two trees from Argana (11 and 20) have fructified for 214 days.
|
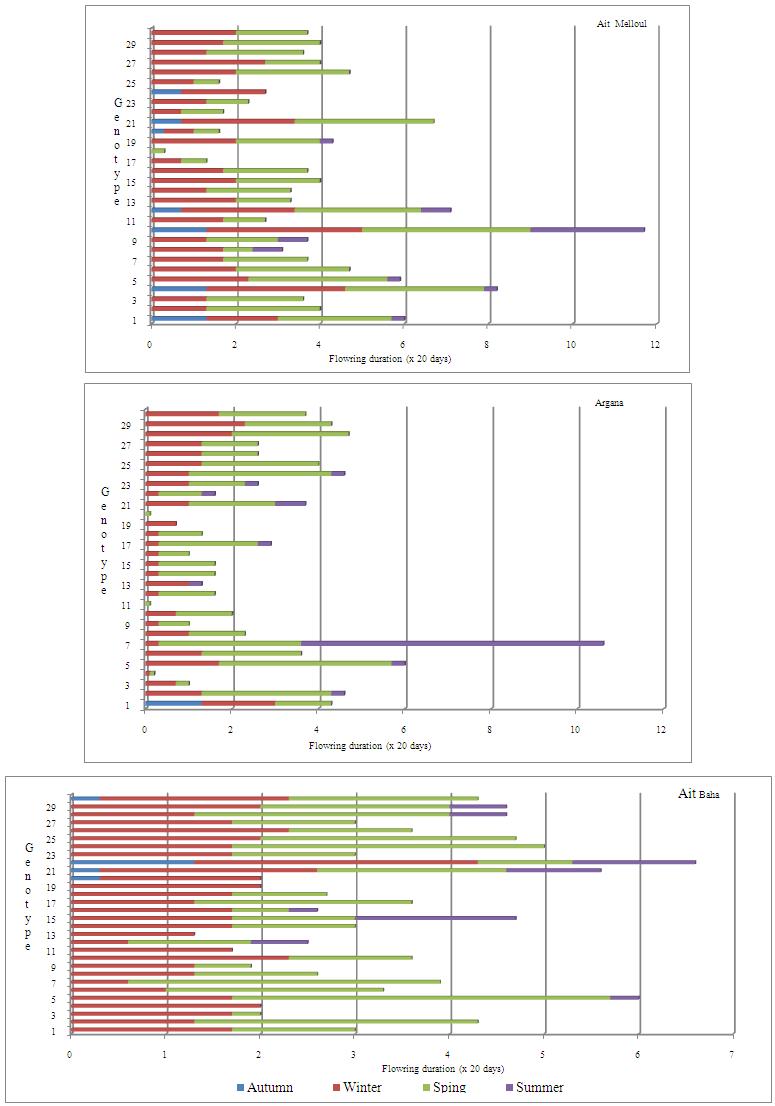 | Figure 2a. Flowering duration (x 20 days) for all genotypes observed in four seasons for three successive years in the sites |
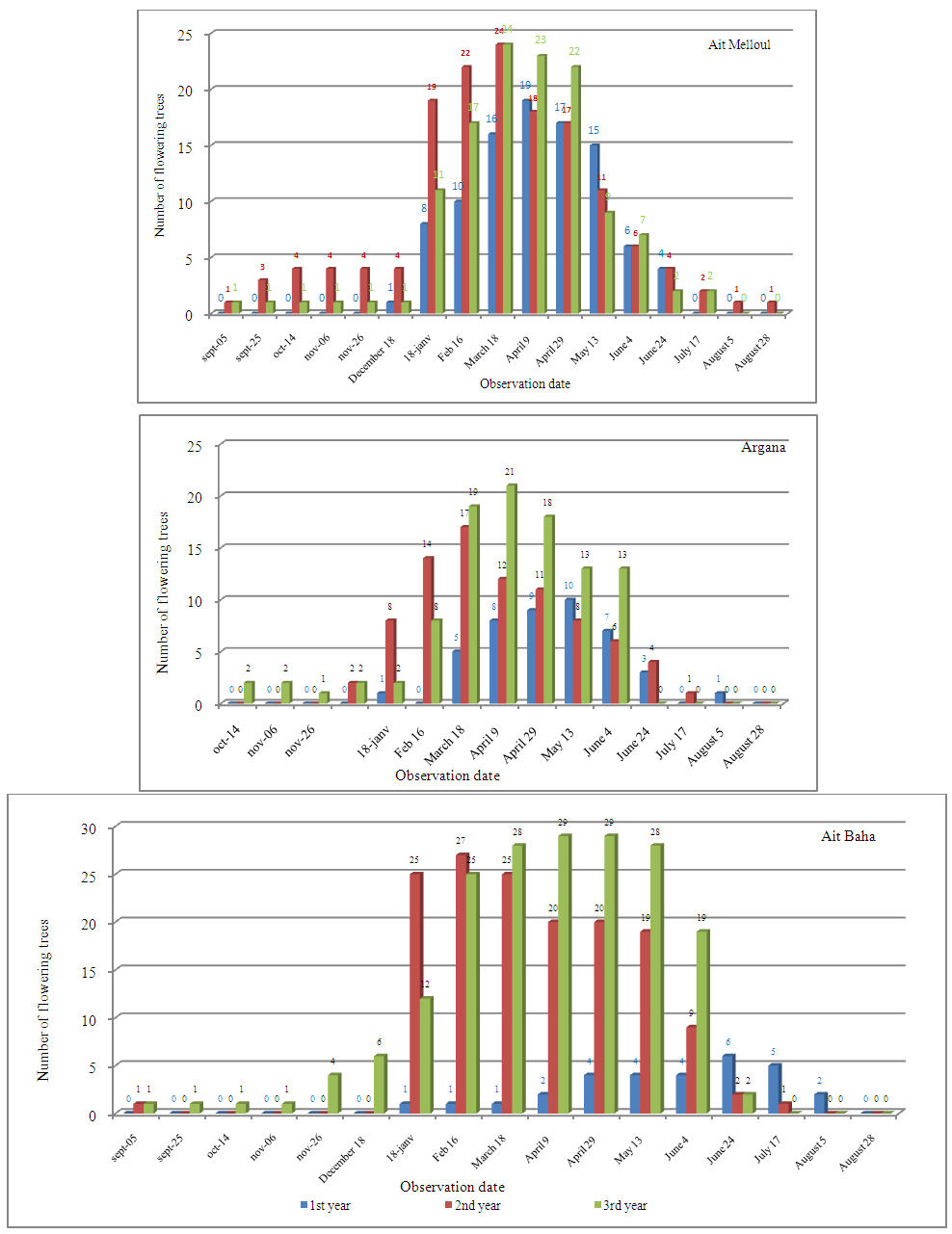 | Figure 2b. Variability in number of flowering trees per year observed in Ait Melloul, Argana and Ait Baha |
 | Figure 3a. Fruiting length (x 20 days) for all genotypes observed in four seasons for three successive years in the sites |
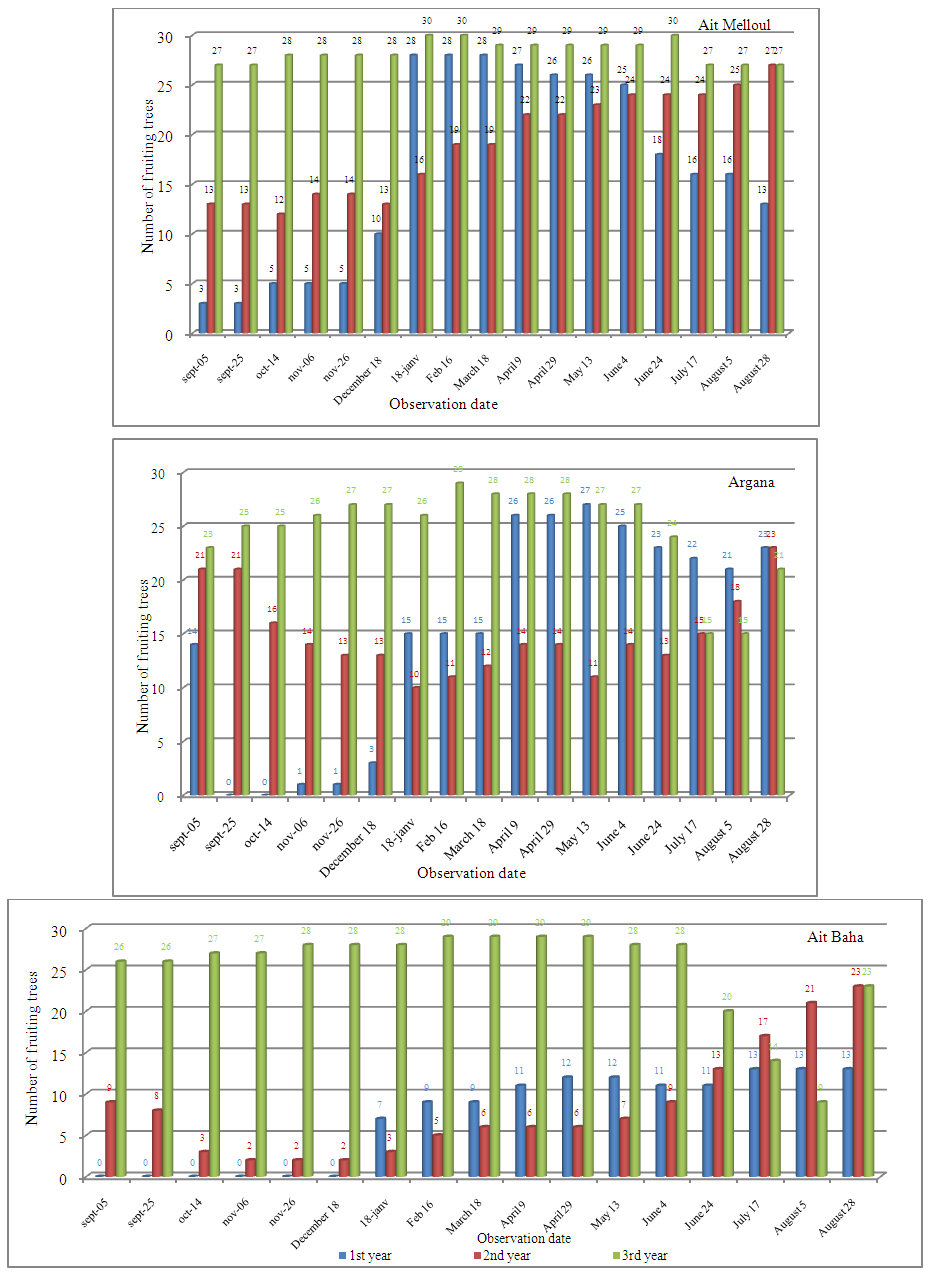 | Figure 3b. Variability in number of fruiting trees per year observed in Ait Melloul, Argana and Ait Baha |
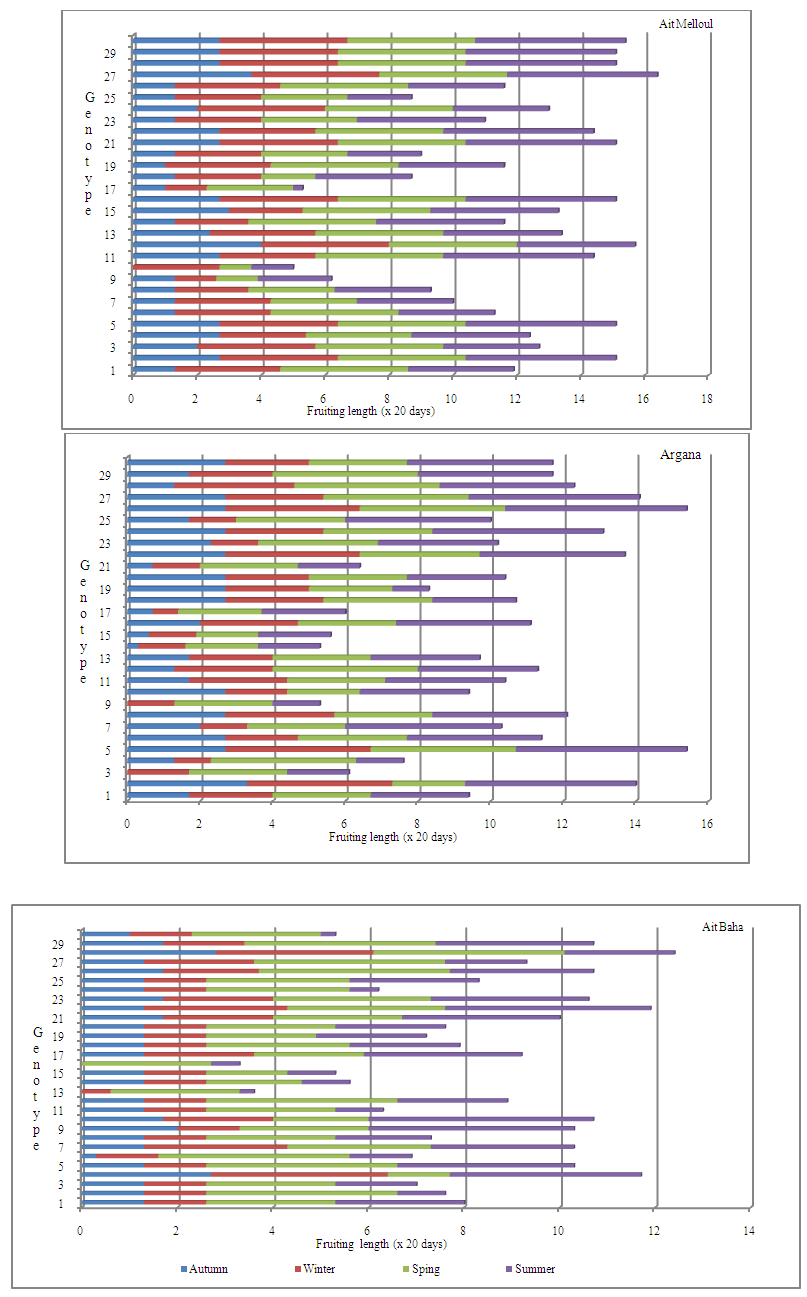
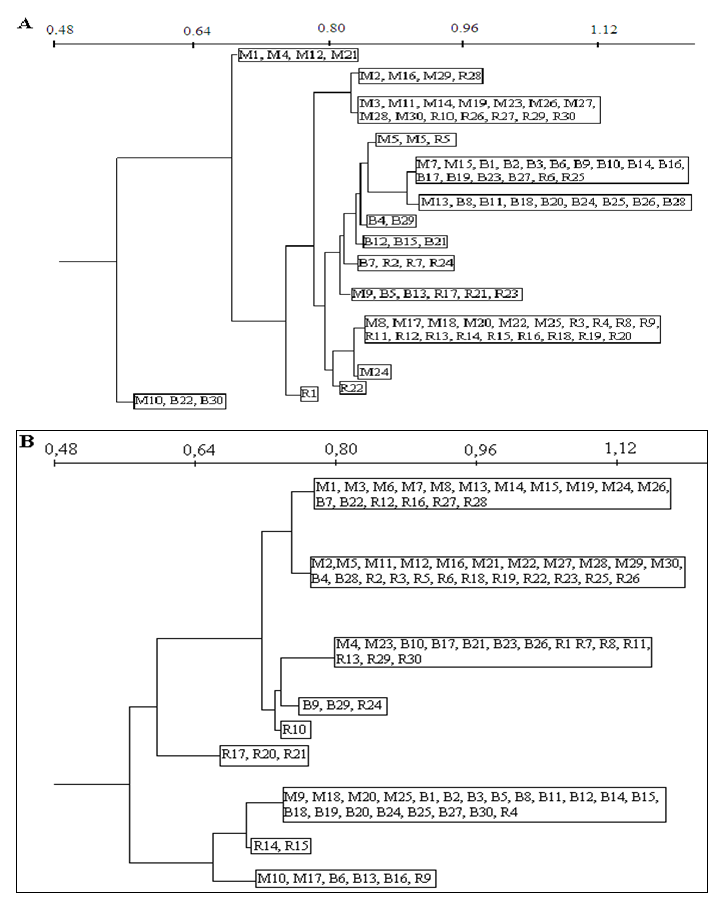 | Figure 4. Classification of genotypes from Ait Melloul (M), Ait Baha (B) and Argana (R) according to flowering (A) and fruiting length s (B) observed during the three consecutive years |
3.2. Frequency of Flowering and Fruiting
3.2.1. Difference between Localities
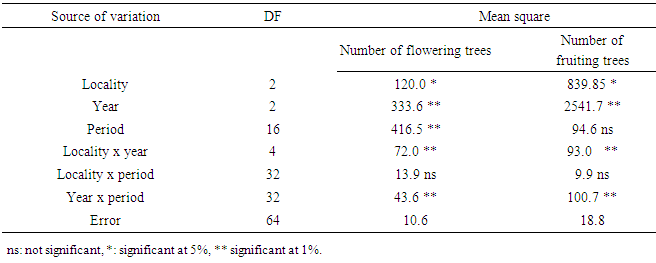
- Locality factor is significant for the number of flowering and fruiting trees (Table 8). During the three years, 81% of the trees from Ait Melloul, 70% from Argana and 69% from Ait Baha have borne flowers. While 92.2% of the trees from Ait Melloul, 93.3% from Argana and only 75.6% from Ait Baha have borne fruits (Figure 5).
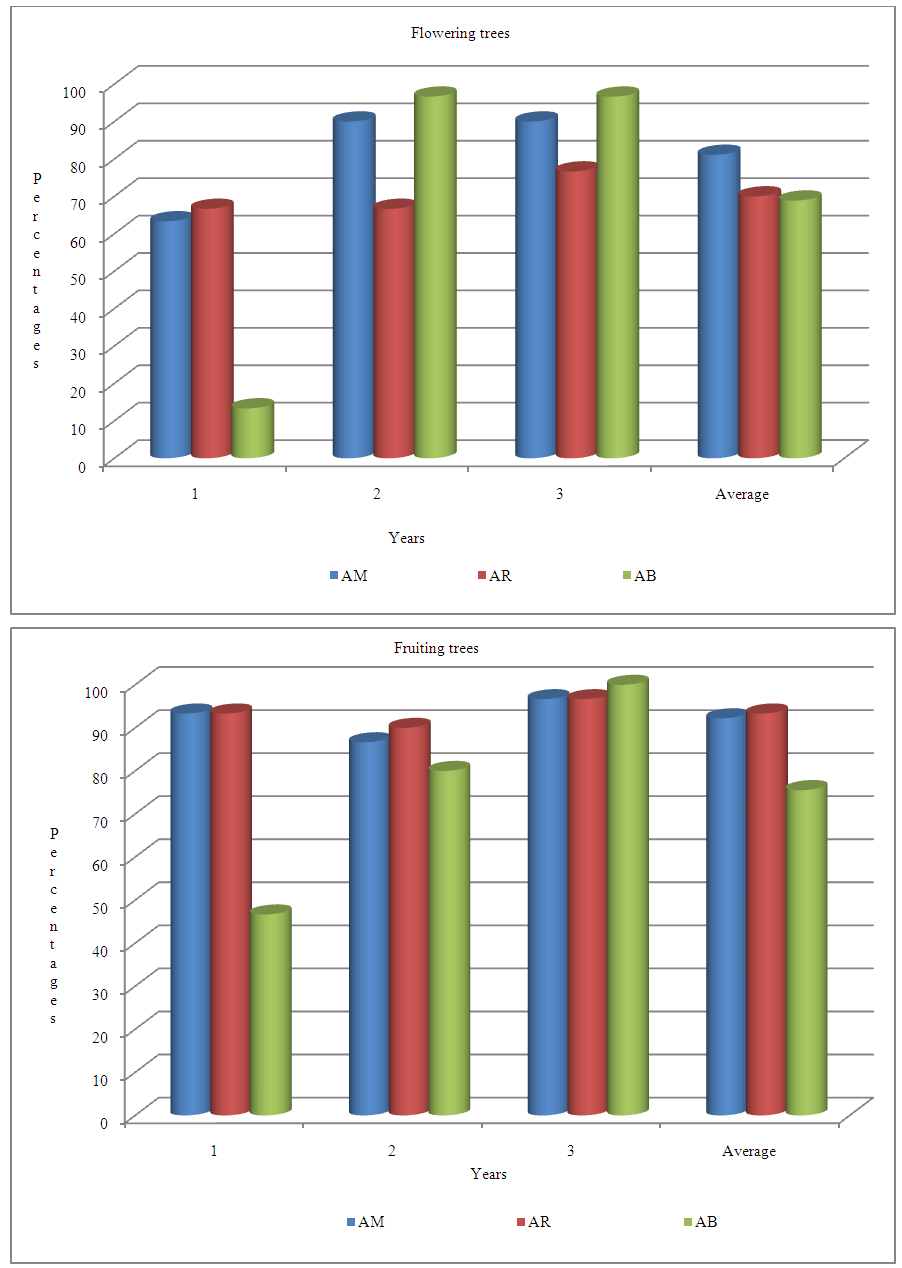 | Figure 5. Percentages of flowering and fruiting trees observed in Ait Melloul, Argana and Ait Baha during the three years |
|
|
3.2.2. Difference between Years
- Year and year x locality interaction affected significantly the number of flowering trees and number of fruiting trees (Table 8). Whatever the locality, the minimum of flowering trees is observed during the dry year (1st year) since only 47.7% of the trees bear flowers. It seems that trees from Ait Baha, the most arid station, are the most affected since only 4 individuals have blooms in short durations (Figure 5, 6). The maximum of flowering trees was recorded during the very humid year (3rd year) (87.8%) and wet year (2nd year) (84.5%). Almost all trees have borne fruits during the 3rd year (97.8%); while the number of fruiting trees was minimal (77.8%) during the 1st year. During the humid year (2nd year) following a dry campaign, about 85.5% have borne fruits.
 | Figure 6. Trees showing simultaneous presence of flowers of the year, fruits from flowering of the year and fruits from flowering of the previous year |
3.2.3. Difference between the Periods of the Year
- The period of year was highly significant for the number of flowering trees but not significant for the number of fruiting trees (Table 8). Locality x period interaction was not significant for both characters. Year x period interaction was highly significant for both characters. During the three years in the three sites, most trees bloom between mid-January and late May with a maximum number of trees in flower between early March and late April (Figure 5). At Ait Melloul, flowering spreads over almost throughout the year for both humid campaigns (2nd and 3rd year) for some trees, the number of flowering trees reached 90% during the most favorable period especially between March and April.
4. Discussion
- The three sites are in semi-arid or arid regions of south west Morocco, characterized by cold winters, followed by hot dry summers. The dry and hot season coincides with the period from May to beginning of September, producing therefore a water deficit during late spring and summer. Water availability varies throughout the year since the first rains begin in September, the latest occurring during the period May-June especially at Argana and Ait Melloul. The maximum temperatures were ranged from 42℃ to 32℃ in August and minimum temperature from 11°C to 14°C in January in Ait Melloul. Inter-annual variability of winter precipitation was higher than temperatures except the first season (Ferradous et al., 1996; Zahidi, 1997; Bani-Aameur and Ferradous, 2001; Zahidi et al., 2013b). In some species, seasonal variation is affecting many aspects of ecological systems, including the seasonal timing (phenology) of biological events. Thus, the present work is an attempt to explore whether the seasonal changes in temperature and precipitation, affects flowering and fruiting phenology in argan in the field. The results indicate that variability of the two traits was the fruit of influence at different levels of geographical origin; year, season of the year, genotype (tree /locality) and genotype x environment interaction (year x tree / locality). During the three years, Ait Melloul population; shows the longest periods of flowering and fruiting well as the number of trees bearing flowers or fruits than the two others localities (Argana and Ait Baha). Argana site of the High Atlas; was the wettest site but characterized by a cold autumn and winter. The decrease of flowering length to only 58 days (approximately one month in less than in Ait Melloul and Ait Baha) during the three years; was probably related to the low temperatures that can induce a slowdown in physiological processes of budding, branches growth, and reproduction process. However, Ait Baha is characterized by its aridity, although flowering length was similar to Ait Melloul with mild temperatures; we notice a significant reduction in periods of the presence of fruits on trees. These periods are reduced to only 154 days (a difference of 88 days). This result will be explained by high temperatures related to hot winds (Chergui) from the south; which may occur 3 to 4 times a year as it is reported in references and cause significant falls of fruits, flowers and leaves (El Aboudi, 1990; Ferradous et al., 1996; Zahidi, 1997). In some fruiting trees, others studies, reported that high daytime temperatures (≥30℃) may negatively affect flower bud initiation and development and then adversely affect flower quality and potential of fruit-setting (Jackson and Hamer, 1980; Caprio and Quamme, 1998). Seasonal variability in temperatures and rainfalls especially in dry campaign (1st) than in humid years (2nd and 3rd); induced a clear reduction in flowering duration, fruiting length and number of trees in bloom and those with fruits. Thus, during the driest and warm year (1st) following a very dry campaign; flowering and fruiting lengths were reduced respectively to 38 and 147 days. But in humid campaign following a very humid year, those periods were extended to 110 days and 300 days. The same effect of drought was also revealed in the three populations, by inducing a significant reduction in number of branches with fruits and the number of fruits on the twigs (Zahidi et al., 2013a). The flowering-fruiting cycle cover a period of 9 to 16 months depending on trees (Bani Aameur et al., 1998; Benlahbil and Bani-aameur, 1999). Thus, in case of drought during the flowering period, fruiting in the following season will be lower. Indeed, during the second year characterized as very humid since accumulated rainfall was about 610.6 mm in Ait Melloul, 1039.5 mm in Argana and 466.2 mm in Ait Baha, but following a dry year (respectively 102.7, 157.8 and 114.3 mm). The periods of flowering and fruiting were limited to only 85 days and 160 days. These rains were beneficial in the contrary in the following year, since the time of flowering and fruiting were remarkable, 72 days of flowering and 153 days of persistent of fruits on the trees in addition. The number of trees has increased also from a minimum of 4 trees with flowers and 14 trees with fruits; to nearly all the trees with flowers and fruits in Ait Baha, the driest site. Sprawl of flowering over a long period represents an additional time which would be beneficial to increase rates of flower visitation for each pollinator, in obtaining pollination and ovules fertilization; although the success of pollination was certainly also affected by the speed with which each pollinator visits flowers (Vazquez et al., 2005). We do not have such data for our ecosystem; but the longevity of the various stages of flower in argan from floral bud open flower was more than 20 days according to tree (Benlahbil, 2004). As well, the presence of fruits on trees will be in favor of fruit ripening and consequently its oil wealth, probably richness by fatty acids e.i. oleic and linoleic acid as reported by Ait Aabd (2013).Our finding indicated high seasonal variability for flowering length and between seasons within the same locality. The full bloom period was observed in winter – spring. It varied from 18 days in winter in Argana, to 42 days in spring in Ait Baha population. Flowering in autumn and summer was less important and interested a limited number of trees. The presence of flowers during the two seasons (autumn and summer) could be explained by the presence in argan of early-trees that bear flowers after the first rains of September and November or late-individuals who can benefit from the late rains recorded in April or May as reported by Bani-Aameur et al. (1998). Most Mediterranean plant species present a single-peaked flowering period in spring. Extended flowering in others species has been traditionally related to the maintenance of favorable and relatively uniform environmental conditions (Petanidou et al., 1995; Bosch et al., 1997; Elzinga et al., 2007). This finding is clearly observed in Ait Baha site; since during dry year (1st year), flowering period was shifted in some individuals between June and August instead of March-April. Under such circumstances, extended flowering seems to be a strategy that minimizes reproductive failure.In agreement, the results of this study suggest that Argania spinosa seems to combine winter and spring flowering with a long period as is the case of several plant species (Rathcke and Lacey, 1985). This bloom is sustained in the summer and fall and may act as an important complement to the main spring reproductive investment. This inter-population and intra-population variability in flowering could be interpreted as a “bet-hedging strategy” for enduring Mediterranean unpredictable and changing environmental conditions and may maximizing the probability of pollination as reported by Sánchez et al. (2012).In the three populations, there were striking differences among genotypes (tree / locality) in both flowering and fruiting lengths. This large variability to drought stress during was manifested by a net reduction in number of flowering trees especially in the first year, since only 26.7% of trees in Ait Baha; 50% in Argana and 66.7% in Ait Melloul site have flowers. But, during wet year (3rd) following a very humid campaign; much than 70% and 95% of trees bear flower and fruit. Ait Baha site was the most affected than Ait Melloul and Argana especially during the dry season (1st). These observations confirm the findings reported by Ferradous et al. (1996) for the frequency of trees bearing fruits; and for fruit yield and fruits component (Zahidi et al., 2014). In this site, where the effects of climatic year was perceived will be considered as a medium for selection of resistant genotypes to drought. Considering this finding; the reproductive stage was the most critical stage for drought stress during crop growth. This factor during reproductive growth usually reduces yield by reducing the number of seeds or seed size (Vieira et al., 1992; De-Souza et al., 1997; Brevedan and Egli, 2003). Thus, in the Green Morocco program, to create varieties by vegetative propagation able to support drought conditions at least in the peripheral regions of the area of argan and ensuring sufficient yields; those genotypes have a good potential regarding this character of drought resistance which can be good sources of germplasm for cultivation.Some genotypes as 10 and 17 from Ait Melloul; 9, 14 and 25 of Argana and 13, 14 and 30 originated from Ait Baha have flowering period less than 120 days but are able to keep the fruit on trees for a period more than 160. Others genotypes have many favorable characteristics in flowering onset, the blooming length, fruiting onset and fruiting duration. Thus individuals (4 and 21) from Ait Melloul; (2, 5 and 26) from Argana; (21 and 22) originated from Ait Baha were able to produce flowers even in very dry year (1st year); and can conserve the formed fruits in the same year. These trees are among individuals able to produce more fruiting branches (main branches or twigs more than two seasons) with three and with four or more fruits and therefore more fruits (Zahidi et al., 2013a). In addition, they can produce fruits even in season of low productivity since yield can reach 21.3 kg (4695 fruits) and about 1075 kg of almonds for oil extraction. This productivity can reach 86.2 kg (20096 fruits) and 5.5 kg of almonds in season higher productivity (Zahidi et al., 2014a). Both trees from Ait Baha, and some genotypes from Ait Melloul and Argana stand out as the most promising since they can produce fruits even in an arid environment and have many favorable fruit characteristics that are commercially important. Hence they can be a good source of germplasm for breeding of argan as a fruit tree for oil production.
5. Conclusions
- Variation in environmental factors (e.g. rainfall, temperature) contributes directly or indirectly to generate heterogeneity on flowering phenology in the three studied populations. Therefore, our finding indicated that temperature and precipitations affects at different levels the flowering duration, fruiting length, number of trees in bloom and number of trees bearing fruits. Inter-population variability (difference between localities) was more important than intra-population variability (difference between trees in the same locality). Some genotypes showed high adaptive plasticity especially in Ait Baha, the dry site. Adapted genotypes to climate changes in arid environments seem to be imperative for improvement in argan. Irregularity of yield is one of the main problems in fruit production. In this regard, selection of individuals will be based on mid to late-bloom, which can ensure high and stable yield under such circumstances and with smaller number of days from bloom to harvest. On other side, controlling cross-pollination would be necessary to increased exchange of genetic information in order to combine the desired characteristics.
ACKNOWLEDGEMENTS
- We gratefully acknowledge anonymous reviewers and office journal which provided helpful comments that greatly improved the manuscript. We thank the Morocco-Germany Co-operative Project ‘Conservation Project and Development the argan forest’ (PCDA-GTZ) and the project Pars-Agro 128 of the Morocan Ministry of Scientific Research for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML