-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(4): 226-239
doi:10.5923/j.ijaf.20150504.03
Physiological Responses of Tomato to Inoculation with Piriformospora indica under Osmotic Stress and Chloride Toxicity
Khalid Al-Absi1, Nofal Al-Ameiri2
1Department of Plant Production, Faculty of Agriculture, Mu’tah University, Karak, Jordan
2Department of Plant Protection and IPM, Faculty of Agriculture, Mu’tah University, Karak, Jordan
Correspondence to: Nofal Al-Ameiri, Department of Plant Protection and IPM, Faculty of Agriculture, Mu’tah University, Karak, Jordan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
A greenhouse to study the response of 'Hildares' tomato (Solanum lycopersicum Mill.) to Piriformospora indica inoculation under osmotic and specific chloride toxicity conditions was conducted in a controlled glasshouse, during the summer season of 2013. The osmotic stress was introduced by irrigation with multicomponent saline water of different electrolyte concentrations (0, 50 and 100 molc/m3) and two ionic compositions, i.e., Cl:SO4 ratio (1:1 or 3:1) at a fixed SAR of 5. The plants were harvested at three times. Irrigation with high osmotic potential solutions (100 molc/m3) significantly reduced fresh and dry weights of both shoots and roots, height of the plants, photosynthesis (Pn) and transpiration (T) rates and leaf water potential (w). Increasing Cl:SO4 ratio of the irrigation water from 1:1 to 3:1 significantly increased the former reductions in the plant parameters. Considerable insignificant variations in growth, leaf water potential and gas exchange of P. indica non-inoculated and inoculated plants were observed. The results have shown insignificant increases in growth of P. indica inoculated plants grown under severe salinity stress, corresponded a significant increase in CO2 assimilation rate in the final harvest and leaf water potential and transpiration rate in the first harvest.The P. indica inoculated plants irrigated with 50 and 100 molc/m3 of Cl:SO4 ratio of 3:1 had significantly higher proline contents than those non-stressed and non-inoculated plants. In conclusion, the study suggests that tomato response to inoculation with P. indica is affected by the single effect of electrolyte concentration an ionic composition of the irrigation water rather than the interaction between the osmotic stress and specific chloride toxicity. For successful irrigation with saline water, attention, as a consequence, should be directed to use saline water that is characterized by high electrolyte concentrations and predominance of chloride ion.
Keywords: Physiological responses, Tomato, Piriformospora indica, Osmotic stress, Chloride toxicity
Cite this paper: Khalid Al-Absi, Nofal Al-Ameiri, Physiological Responses of Tomato to Inoculation with Piriformospora indica under Osmotic Stress and Chloride Toxicity, International Journal of Agriculture and Forestry, Vol. 5 No. 4, 2015, pp. 226-239. doi: 10.5923/j.ijaf.20150504.03.
Article Outline
1. Introduction
- Environmental stresses have a profound effect on the distribution of natural and cultivated vegetation. Availability of quality irrigation water is the main limiting factor for productive agriculture in many countries of the arid and semi-arid regions. Agriculture in Jordan is restricted by a shortage of land receiving good rainfall. More than 90% of the country's 9 million hectares of land receives less than 200 mm annual rainfall. Water scarcity; nearly always aggravated by poor quality, push planers, decision makers and water users more and more to augment water supplies to such non-conventional sources as saline water and wastewater [4]. The inhibitory effect of saline solutions on plant growth is generally attributed to two main reasons, namely: osmotic and/or specific-ion effects [20].Tomato is one of largest and most valuable vegetable crops in many countries. It is a common vegetable species in the Mediterranean basin. It is known to be moderately salt-tolerant. The threshold value of its salinity tolerance is often used as a standard for evaluating irrigation water quality and reflects the response of plants to the average root zone salinity. Much of the research quantifying the salt tolerance of plant species has been based on field studies and refers to plants treated with saline solution of diverse chemical characteristics, thus making comparative interpretation of results difficult. Few articles on the effect of osmotic potential of multicomponent solutions on vegetative growth and mineral content of tomato have been published and most authors have used a single salt, usually NaCl [4, 5, and 6]. However, the use of single salt solutions does not reflect the composition of typical irrigation water. The tolerance response to salt stress differs greatly among various tomato cultivars [5, 11, 14]. Several selection criteria have been proposed for selecting genotypes based on their performance in stress and non-stress environments. Differences among species in leaf morphology, leaf water potential, osmotic potential, photosynthesis and stomatal conductance are involved, to varying extents, in determining how a species responds to salinity. The main consequence of osmotic stress is decreased growth and development caused by reductions of leaf area, dry matter production, decline in plant water status and anatomical changes [1, 15]. Piriformospora indica is a root endophytic fungus of the order Sebacinales (Basidiomycota) with a wide host range and has enormous potential for growth promotion of plants by colonization of their roots [29]. In contrast to arbuscular mycorrhizal fungi, P. indica can be easily grown on synthetic media allowing for large-scale propagation and a possible use in plant production [16]. Growth of this root endophyte is restricted to the cortical tissue of the root, predominantly in the differentiation and the root hair zone [12]. Apart of promoting growth, it could be shown in various plants that root colonization with P. indica increases the resistance of plants against root and leave pathogens [28, 19, and 25]. The ability of P. indica to improve abiotic tolerance of plants is well documented [16]. Most important for the proposed project, it has been found in experiments with barley that P. indica-inoculated plants are more tolerant to salt stress [29, 31], Root colonization by P. indica attenuated the NaCl-induced lipid peroxidation, metabolic heat efflux and fatty acid desaturation in plants under abiotic stress [7, 26]. The detrimental effects of moderate salt stress on barley plants grown in hydroponic culture is completely eliminated by P. indica colonization, as shown by the fact that colonized plants produce higher biomass than do non-stressed control plants under these conditions [22]. The endophyte seemed to over-compensate the salt-induced inhibition of leaf metabolic activity [7]. P. indica colonization leads to clear changes in fatty acid composition in barley leaves. This effect may display a symbiotic adaptive strategy mediated by the endophyte to cope with salt stress at stress environments [17].In as series of experiments carried out at the IGZ, it has been shown that P. indica can be also applied to tomato production systems. The endophyte limited disease severity caused by Verticillium dahliae and improved growth and yield of tomato in hydroponic cultures resulting in increased biomass of leaves, increased fruit biomass and [13].The objectives of this work was to study the response of tomato vegetative growth and related parameters, photosynthesis, transpiration, water use efficiency, water relations and proline content to Piriformospora indica inoculation under osmotic stress and specific chloride toxicity of multicomponent hydroponic solutions (comprising Na, Ca, Mg, K, Cl, SO4, and HCO3).
2. Materials and Methods
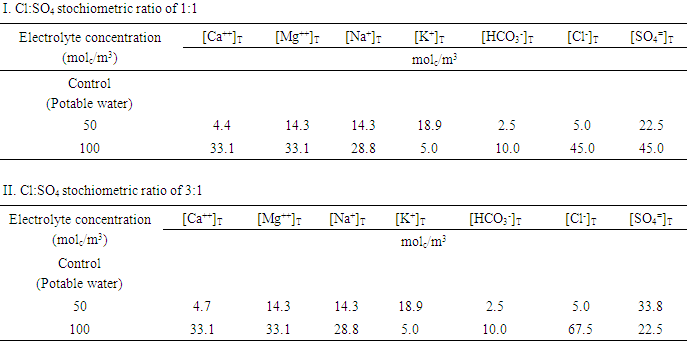
- 1. Plant material and growth conditions A greenhouse experiment to study the response of tomato (Solanum lycopersicum Mill.) to Piriformospora indica inoculation under salt stress conditions was conducted in a glasshouse, in which temperature and ventilation can be controlled, located at the Leibniz-Institute of Vegetable and Ornamental Crops, Großbeeren during the period June, 24 – September, 20 2013. Hildares tomato cultivar was used. Hildares was used as a model organism because it is susceptible to the endophytic fungi. Uniform two weeks old transplants were planted in July, 9 2013 in a hydroponic culture in 10L buckets containing half strength of the nutrient. Macro- and microelements were added at the 0.5-strength Hoagland nutrient concentration once every 3 days for 10 days before treatments application began. Tomato plants were held in place using polyester plugs inserted into holes and were established in the hydroponic system. Aeration was maintained through an aquarium bubble stone by an oilless diaphragm pump. The roots were suspended in the nutrient solution, and the stem was wrapped with sponge plugs to hold the plants firmly.2. Piriformospora indica treatmentsTwo P. indica treatments were applied, inoculated or none inoculated with a mixture of spores and hyphae according to [10, 13]. 3. Salinity treatmentsThe saline irrigation water were prepared by mixing chloride, sulfate and bicarbonate salts of sodium, calcium and magnesium with distilled water in order to obtain the desired multicomponent electrolyte solution, typical of most saline irrigation water compositions of Jordan. Tap water supplemented with full strength Hoagland solution was used as control. In addition to the control treatment, the irrigation water treatments included 12 combinations (Table 1) of saline water compositions having:A: Total electrolyte concentrations of 0, 50 and 100 molc/m3 (osmotic stress).B: Cl:SO4 stochiometric ratio of 1:1 or 3:1 (chloride toxicity) C: Constant stochiometric ratio of 1 for Ca:Mg.D: Constant level of SAR=5Calculations were done using a multicomp computer program [2]. Table 1 shows the chemical composition of the irrigation water that was used throughout the research. Ionic speciation of the above solutions was performed using the WATEQ program [27] at pH 7 to keep track of precipitation of gypsum and calcite.The plants were harvested at three times, as follows:First harvest: August 20 2013Second harvest: August 26 2013Third harvest: September 2 2013
|
3. Measurements
- At termination of the investigation, plant height, as a growth parameter was recorded from individual plants at certain intervals. Fresh weight of vegetative parts and roots was measured. Dry mass of plants was determined after oven drying at 70C until a constant weight is obtained. Leaf water potential of four plants of each treatment was measured before the treatments imposed and during the experimental period using a pressure bomb. It was measured on the most recent fully expanded, mature leaf. The measurements were taken on two fully expanded leaves per plant. Measurements were done between 9:00 and 14:00. Gas exchange parameters [CO2 assimilation rate and transpiration] were measured during stress periods (just before irrigation) on intact leaf (fully expanded) from the top of each plant using a portable steady state infrared gas analyzer (LI-6400, LI-COR, Lincoln, NE, USA). Three measurements were made during the course of the experiment 5, 6 and 7 weeks after treatments initiation. The measurements were taken between 09:00 to 13:00. Leaf proline content was calorimetrically estimated in fresh leaf samples ten weeks after beginning of the investigation, according to the method of [8].
4. Experimental Design
- The experimental design was factorial combination of treatments, arranged as a randomized complete block (RCBD) design. Each treatment was replicated four times. The data were subjected to analysis of variance (ANOVA) using the general linear model (GLM) of SAS (SAS Institute, Cary, NC) according to [24). Treatment means were compared using Least Significant Difference (LSD) test at 5% level.
5. Results and Discussion
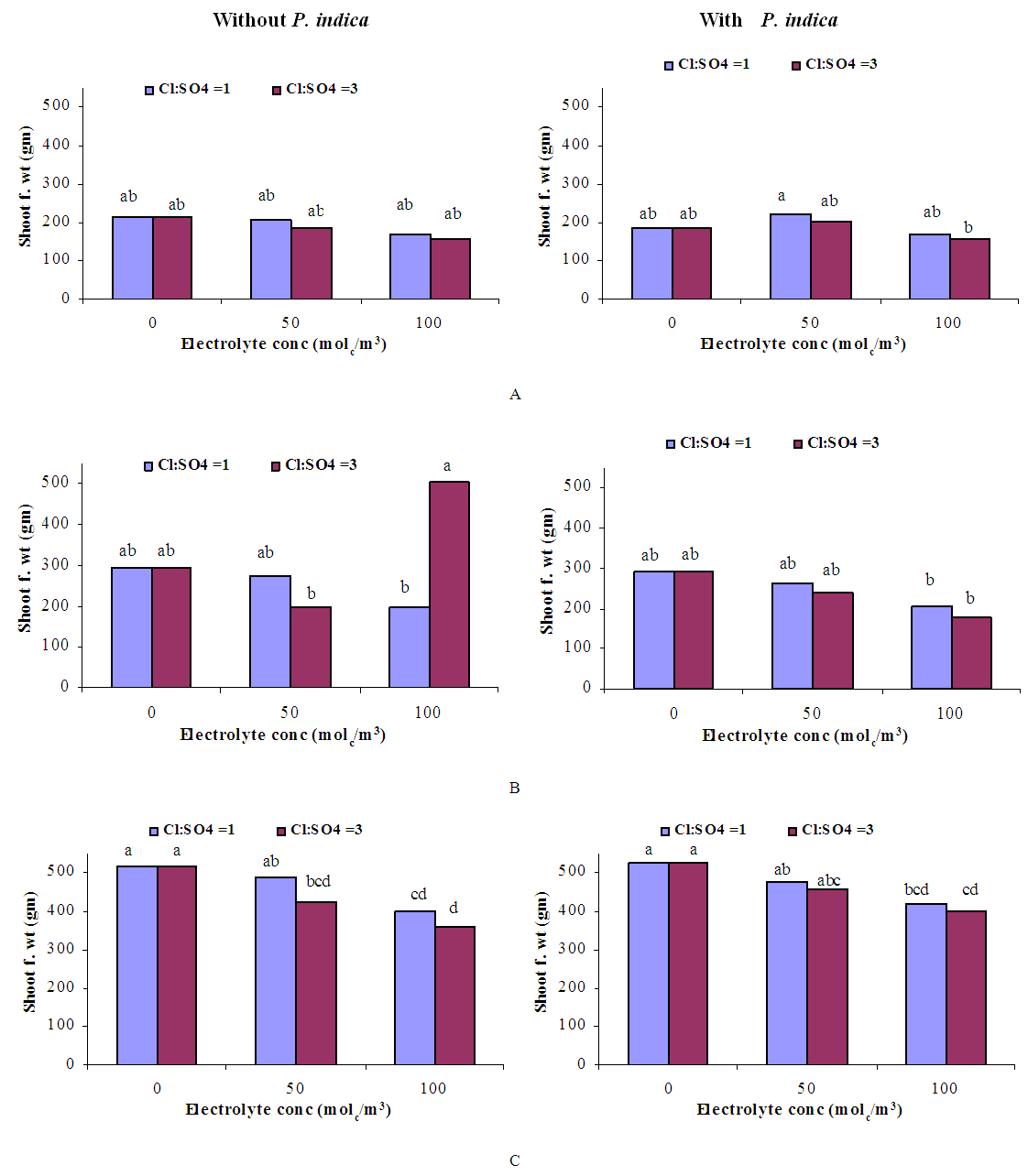
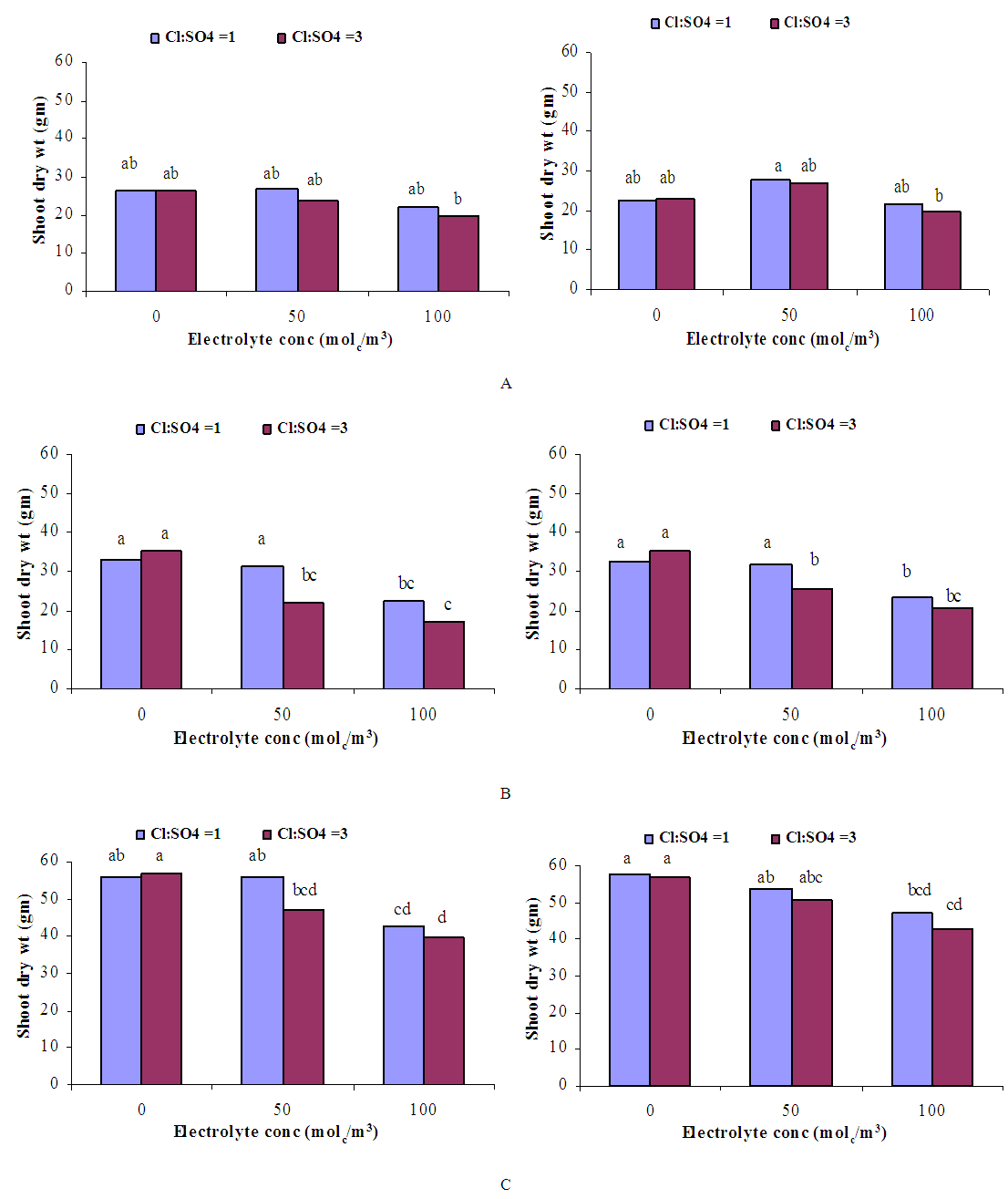
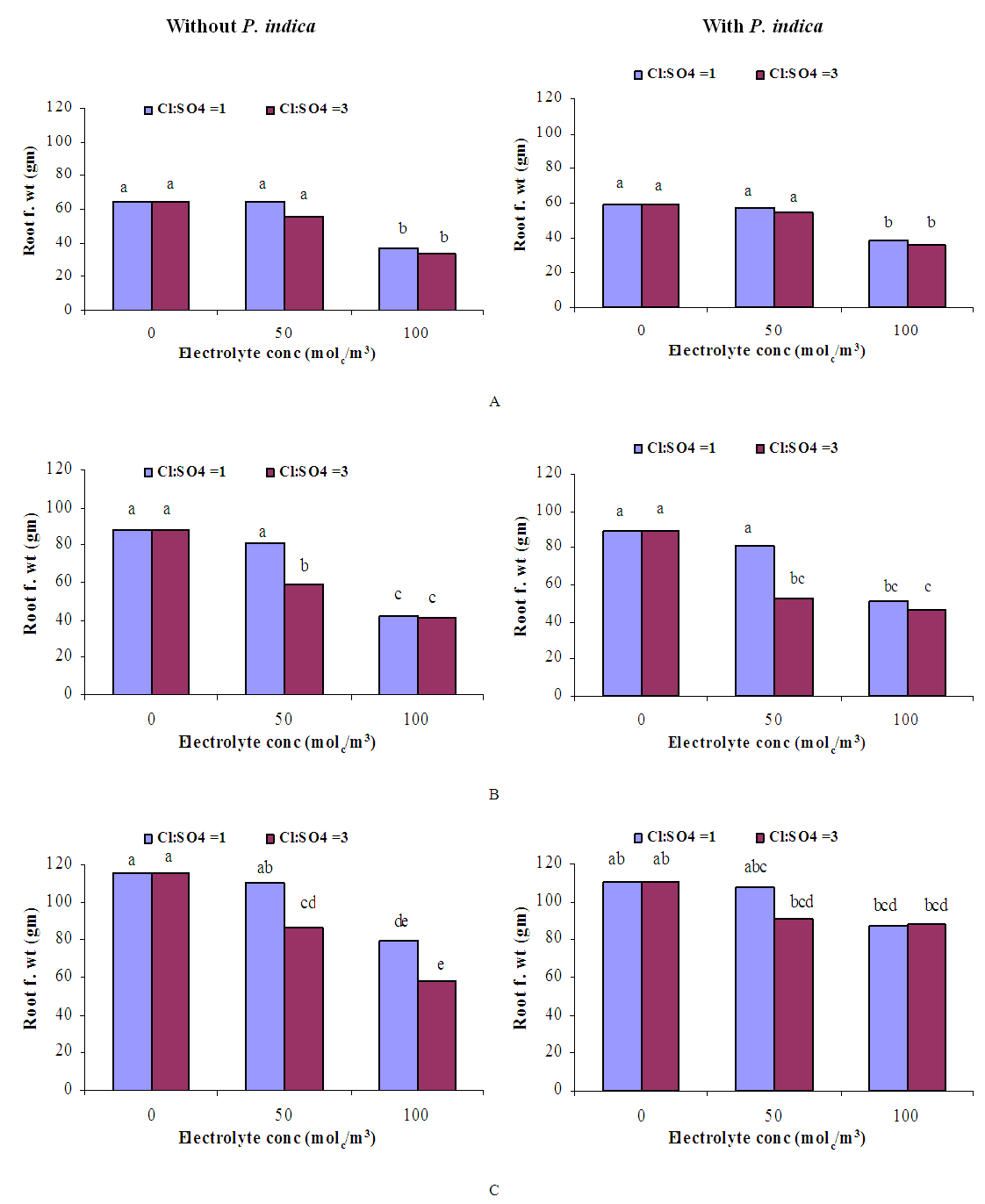
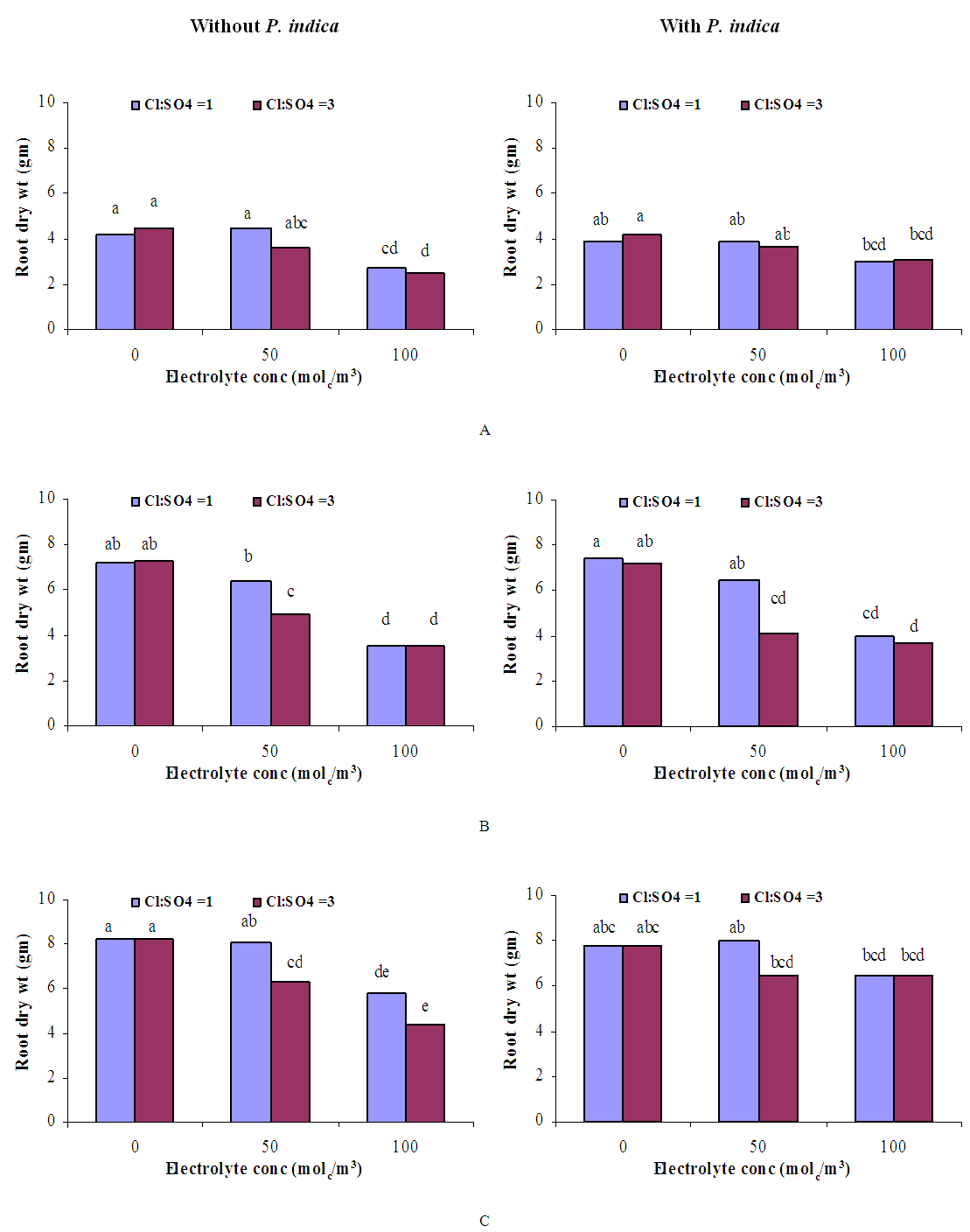
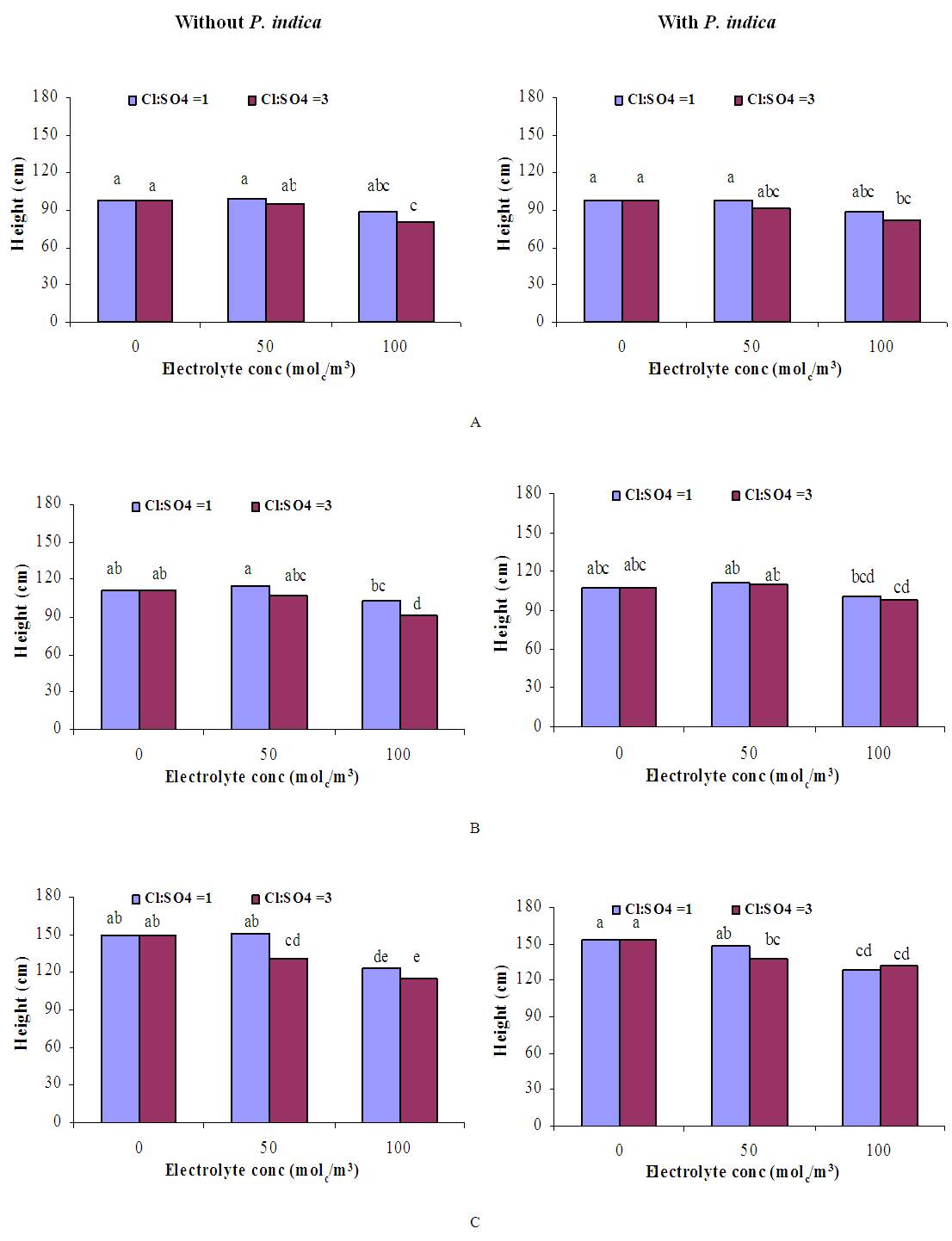
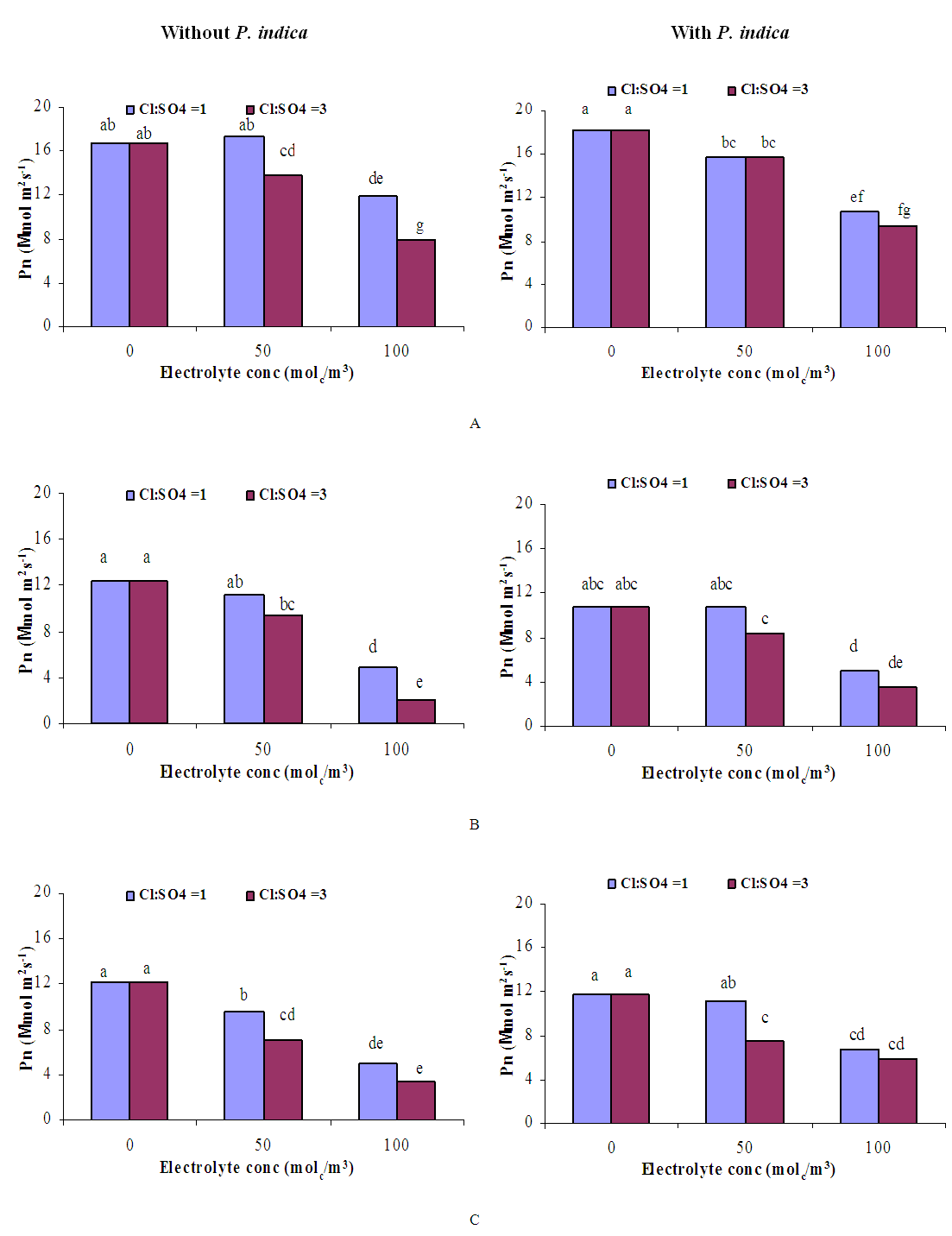
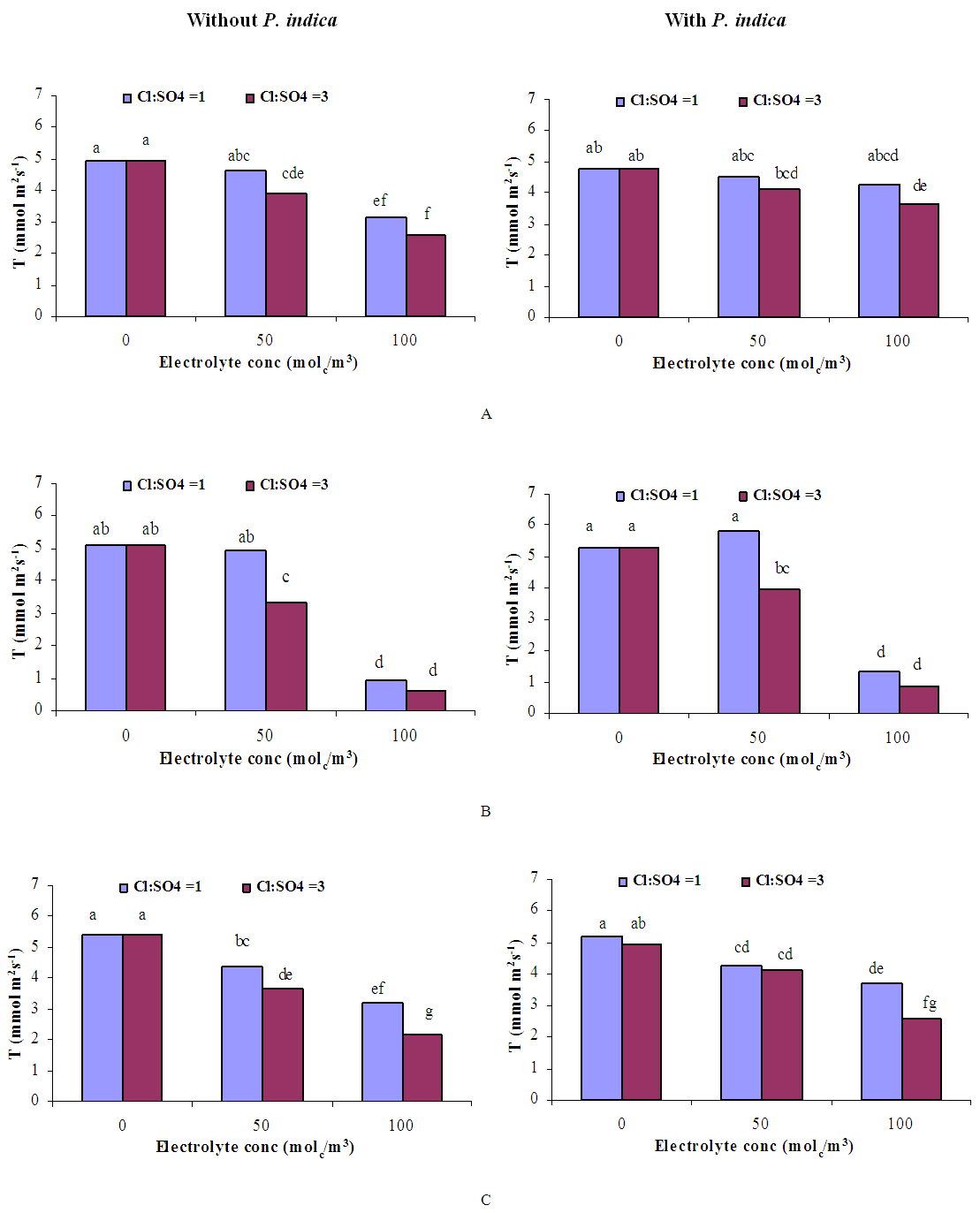
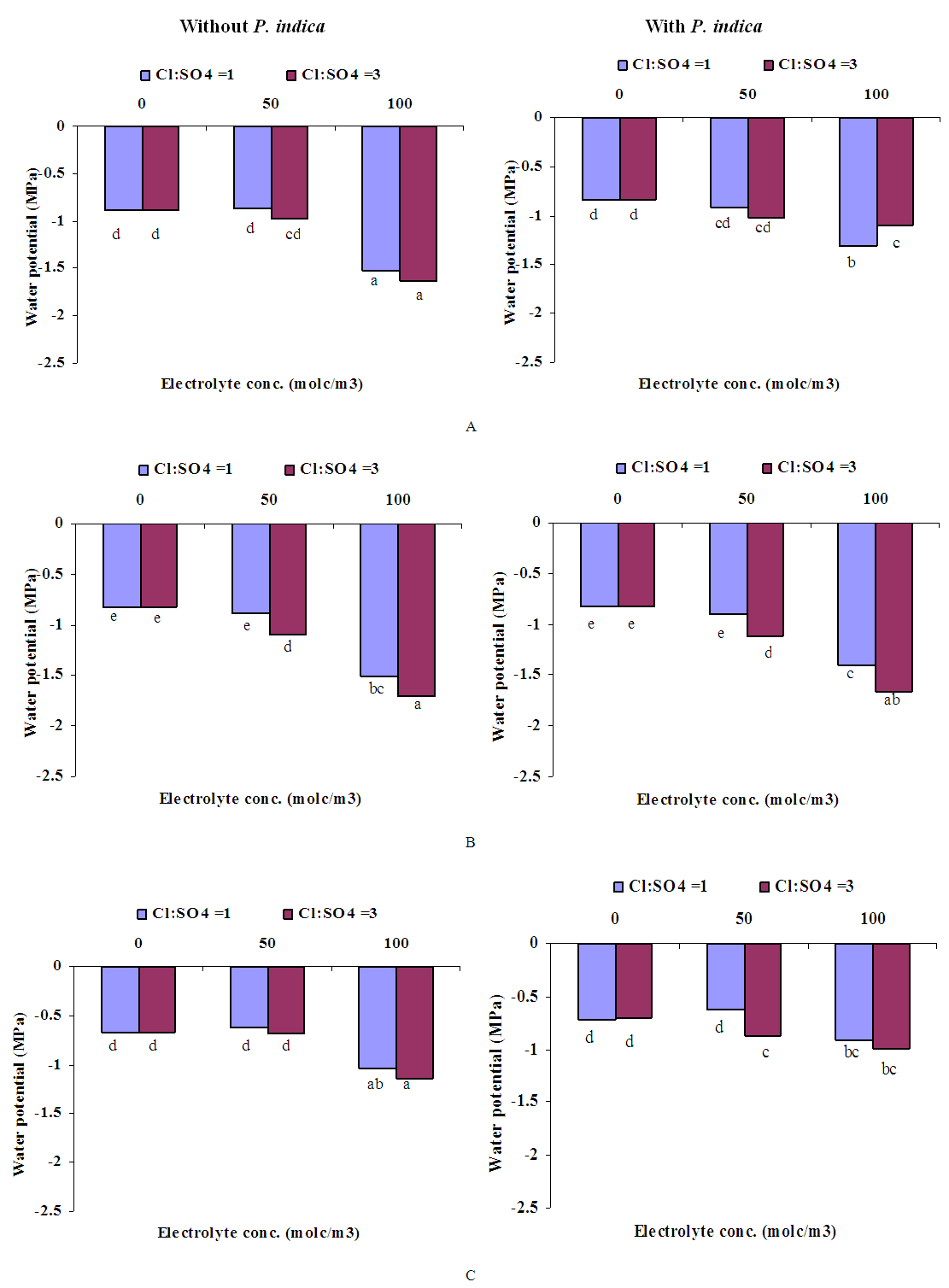
- GrowthGenerally, in all harvests, the growth of tomato plants was greatly affected by the salt stress (Figures 1-5). Although it was not significant, inoculation of tomato plants with Piriformospora indica increased fresh and dry weights of the shoot and root. No interactive effect of the electrolyte concentration x ionic composition of the irrigation water x inoculation with P. indica on plant height fresh and dry weight of shoot and root was found. This result about the influence of interaction between the endophyte and biotic stress is in accordance with that obtained on barley [3]. The limited effect of P. indica on vegetative growth was probably due to the extent and mode of colonization [13, 21].Irrespective of inoculation with Piriformospora indica and electrolyte concentration of the irrigation water, data presented in figures 1-5 reveal a pronounced response to salinity and ionic composition of the irrigation water. The inhibitory effect was clearer at the final harvest. The results elucidate that salinity, especially at higher concentrations, markedly reduced plant height fresh and dry weight of shoot and root. There were no significant differences between the control and 50 molc/m3 treatments. Plant height, shoot and root fresh weights were significantly reduced only when using an electrolyte solution of 100 molc/m3. Visible symptoms of chloride toxicity and osmotic stress such as leaf wilting and burning of leaf edges were not noticed until 25 days after imposing the non-inoculated plants to 100 molc/m3 and Cl:SO4 ratio of 3:1. On the contrary, the symptoms appeared after 42 days on the inoculated plants.In the third harvest, increasing the salinity of irrigation water to 100 molc/m3 reduced plant height by 17.7, fresh and dry weight of shoot by 24.7% and 24.1 and root by 30.3 and 27.6, respectively. The higher Cl:SO4 ratio (3:1) significantly reduced young shoot length. This result highlights the importance of the specific effect of chloride on length of shoots. Increasing the chloride fractional negative charge in the multicomponent irrigation water from 1:1 to 3:1 caused a clear reduction in the growth of tomato shoots and roots. The current study indicates that high chloride proportions in saline water add to their adverse effects on plant growth by toxic accumulation of chloride. For successful irrigation with saline water, attention, as a consequence, should be directed to using saline water that is characterized by predominance of chloride ion such as the groundwater of some areas of Jordan in which the chloride fractional negative charge tended to increase to an average steady-state value of about 0.8. Earlier studies indicated that root colonization by P. indica improved plant biomass in tomato [13] and wheat [19] and barley [29]. The ability of P. indica in improving biomass of salt-stressed plants is due to its power to alleviate the salt-induced inhibition of leaf metabolic activity [7]. Furthermore, the enhancement of root was attributed to either on fungal production of phytohormones like the auxin indole acetic acid [23] or to a transiently altered expression levels of gibberelic acid biosynthesis genes and abscisic acid responsive genes [18].Gas exchangePrior to initiation of salinity and P. indica treatments, the photosynthesis (Pn) and transpiration rates (T) rates were 16.25 µmol CO2 m-2 s-2 and 4.88 mmol H2O m-2 s-1.Regardless of root colonization by P. indica and ionic composition of the irrigation water, the change in electrolyte concentration of irrigation water resulted in a highly significant decrease in photosynthesis and transpiration rates of tomato plants (Figures 6 and 7). The 50 molc/m3 treatment was not able to significantly reduce Pn and T rates. However, 100 molc/m3 drastically reduced the number of shoots.The ionic composition of the irrigation water, in terms of Cl:SO4 ratio, had a significant effect on Pn and T rates in all harvests. However, the inoculation with P. indica has a significant effect on T rate in the first harvest and Pn rate of the plants in the third harvest. P. indica inoculated plants irrigated with 100 molc/m3 of Cl:SO4 ratio of 3:1 had significantly higher Pn values than those non-inoculated plants. In other words, the inoculation of tomato plants with P. indica improved the tolerance of plants irrigated with high toxic of chloride and osmotic potential. On the other hand, the inoculation with P. indica insignificantly reduced T rates of the plants irrigated with high toxic of chloride and osmotic potential. These results suggest that this endophyte may act as biofertilizer and biocontrol agent. Water relationsData presented in table 8 indicate that leaf water potential
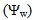 of tomato plants was lower in salt-stressed plants compared to non-stressed plants (Figure 8). The highest electrolyte concentration of irrigation water (100 molc/m3) and the Cl:SO4 ratio of 3:1 strongly reduced the
of tomato plants was lower in salt-stressed plants compared to non-stressed plants (Figure 8). The highest electrolyte concentration of irrigation water (100 molc/m3) and the Cl:SO4 ratio of 3:1 strongly reduced the 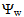 of the leaves. The root colonization with P. indica significantly reduce the leaf
of the leaves. The root colonization with P. indica significantly reduce the leaf 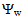 of the plants harvested on August 20 2013, while it was not able to significantly reduce the leaf
of the plants harvested on August 20 2013, while it was not able to significantly reduce the leaf 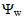 of the plants harvested on August 26 or September 2. In the presence of P. indica, irrigation with chloride toxicity and high osmotic potential was found to induce a general reduction in the leaf water potential of tomato plants harvested early on August.Leaf proline contentLeaf proline content of non-inoculated plants was not significantly affected by electrolyte concentration and ionic composition of the irrigation water (Figure 9). However, the P. indica inoculated plants irrigated with 50 and 100 molc/m3 of Cl:SO4 ratio of 3:1 had significantly higher proline contents than those non-stressed and non-inoculated plants. The increase in proline has been previously reported to involve in protection against oxidative damage during dehydration [28]. In the current investigation, because proline increased P. indica inoculated plants irrigated with 50 and 100 molc/m3, its accumulation could be considered as a symptom of salt stress and P. indica inoculation rather than an indicator of tolerance to stress.
of the plants harvested on August 26 or September 2. In the presence of P. indica, irrigation with chloride toxicity and high osmotic potential was found to induce a general reduction in the leaf water potential of tomato plants harvested early on August.Leaf proline contentLeaf proline content of non-inoculated plants was not significantly affected by electrolyte concentration and ionic composition of the irrigation water (Figure 9). However, the P. indica inoculated plants irrigated with 50 and 100 molc/m3 of Cl:SO4 ratio of 3:1 had significantly higher proline contents than those non-stressed and non-inoculated plants. The increase in proline has been previously reported to involve in protection against oxidative damage during dehydration [28]. In the current investigation, because proline increased P. indica inoculated plants irrigated with 50 and 100 molc/m3, its accumulation could be considered as a symptom of salt stress and P. indica inoculation rather than an indicator of tolerance to stress.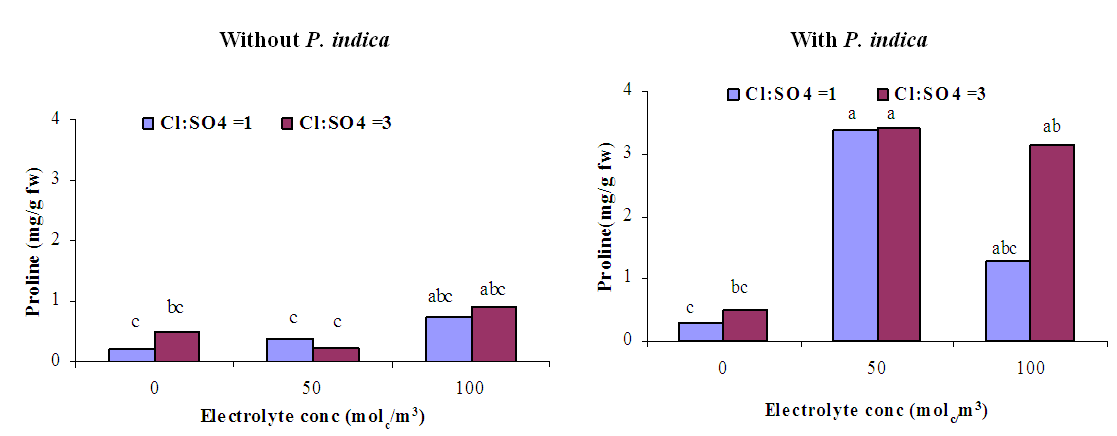 | Figure 9. Influence of irrigation water salinity of two ionic compositions and root colonization with P. indica on leaf proline content of ' Hildares' tomato plants harvested on September 2, 2013 |
6. Conclusions
- In conclusion, results of the current study have shown that for successful irrigation with saline water, attention, as a consequence, should be directed to use saline water that is characterized by high electrolyte concentrations and predominance of chloride ion. A clear increase in leaf proline content and a considerable decline in vegetative growth parameters of olives as well as photosynthesis, transpiration rates and leaf water potential were corresponded with irrigation with high osmotic potential solutions. The decline was more severe with irrigation water having chloride toxicity (Increasing Cl:SO4 ratio of the irrigation water from 1:1 to 3:1). Considerable insignificant variations in growth, leaf water potential and gas exchange of P. indica non-inoculated and inoculated plants were observed. The P. indica inoculated plants irrigated with 50 and 100 molc/m3 of Cl:SO4 ratio of 3:1 had significantly higher proline contents than those non-stressed and non-inoculated plants. The study suggests that tomato response to inoculation with P. indica is affected by the single effect of electrolyte concentration an ionic composition of the irrigation water rather than the interaction between the osmotic stress and specific chloride toxicity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML