-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(3): 199-204
doi:10.5923/j.ijaf.20150503.04
Contributions of Microorganisms to Soil Fertility in Adjacent Forest, Fallow and Cultivated Land Use Types in Nsukka, Nigeria
Asadu C. L. A., Nwafor I. A., Chibuike G. U.
Department of Soil Science and Land Resources Management, University of Nigeria, Nsukka, Nigeria
Correspondence to: Asadu C. L. A., Department of Soil Science and Land Resources Management, University of Nigeria, Nsukka, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Soil chemical properties and microbial populations were determined in this study in order to compare the contributions of microorganisms to soil fertility in three land use types in an Ultisol of southeastern Nigeria. A total of 27 samples were collected from 0-20 cm depth as follows: 21 samples from a fallow land (3 replicates from 7 plots) that has been under 10-year fallow, three samples from an adjacent forestland and finally, three samples from an adjacent cultivated land owned by a farmer. These samples were air dried, passed through a 2-mm sieve before soil properties were determined following standard methods. Fresh soil samples were used to determine the number of soil microorganisms via the dilution spread plate technique using the nutrient agar for bacteria and potato dextrose agar for fungi. Results showed that the forest and fallow lands had significantly (P < 0.05) lower mean pH value, available P, exchangeable K and Na, but significantly higher exchangeable H and bacteria population than the cultivated land. The mean exchangeable Ca was significantly (p < 0.05) higher in the cultivated land than in the fallow land but similar to that from the forestland. The fungi population was significantly (p < 0.05) higher in the forestland than in others which are similar statistically. The mean soil organic matter, total N, exchangeable Mg, exchangeable Al and CEC were statistically similar in all the land use types. Contributions of microorganisms to soil fertility were generally more in the uncultivated lands, an indication that tillage operations may have affected the microbial populations. Significant correlations (p < 0.05) obtained between some soil chemical properties and microbial densities signify important roles microorganism play in soil nutrient build up.
Keywords: Bacteria, Fungi, Soil fertility, Forestland, Fallow land, Cultivated land, Ultisol
Cite this paper: Asadu C. L. A., Nwafor I. A., Chibuike G. U., Contributions of Microorganisms to Soil Fertility in Adjacent Forest, Fallow and Cultivated Land Use Types in Nsukka, Nigeria, International Journal of Agriculture and Forestry, Vol. 5 No. 3, 2015, pp. 199-204. doi: 10.5923/j.ijaf.20150503.04.
Article Outline
1. Introduction
- Microorganisms are responsible for most biological transformations that result to the development of nutrients in the soil [1]. They influence several soil functions and are key indicators of soil quality. These organisms ensure the continued existence of nutrients in the soil. Management of these nutrients through sustainable use of soil resources is a requisite for successful agriculture [2]. Identifying and quantifying soil microorganisms may be one way of determining soil nutrient status [3]. This could help in the maintenance of soil nutrients for improved crop productivity. Several factors including the physical and chemical properties of soils can influence the type of species, number and activities of microorganisms in a soil [4]. Studies have shown that in addition to environmental factors such as temperature, moisture and CO2 levels, soil physical disturbance (structure), soil pH and other chemical properties are major determinants of soil microbial community structure [5-8]. Land use and soil management practices can influence soil nutrients through processes such as mineralization, oxidation, leaching and erosion [9, 10]. This may affect the presence and activities of soil microorganisms and hence soil fertility. For instance, intensive tillage operations common in continuously cultivated soils may lead to increased decomposition / mineralization of available nutrients which may result to loss of nutrients from these soils. Studies examining how different land use types influence the contributions of soil microorganisms to soil fertility are uncommon in tropical soil studies. Thus, the main objective of this research was to examine how three different land use types influence the contribution of microorganisms to soil fertility in Nsukka, Nigeria. Interrelationships between soil chemical and microbial properties were also compared.
2. Materials and Methods
2.1. Description of Study Area
- The study was conducted in Edem Nru, Nsukka located within latitude 6°52′3″N and longitude 7°23′2″E. Nsukka is in the derived savannah area of southeastern Nigeria with a mean annual rainfall of about 1550 mm. It has a mean annual temperature of 22℃ (minimum) and 30℃ (maximum). Relative humidity and height above sea level are 60% and 447 m, respectively [11]. Soils of the area are formed from the disintegration of false-bedded sandstone and upper coal measures which give rise to sandy and clayey soils respectively [12, 13]. These soils have been classified as Ultisol [14]. They are usually prone to erosion and leaching as a result of exposure to rainfall of high intensity prevalent in the area.
2.2. Land Use Types
- The land use types examined were forestland, fallow land, and cultivated land. The forestland is a natural forest which has not been disturbed for at least 100 years. Although shrubs and climbers are present in the forest, the major plant species are trees such as African mango (Sphenostylis stenocarpa), bamboo (Bambusa vulgaris), oil bean (Pentaclethra macrophylla), and oil palm (Elaeis guineensis). The fallow land had been previously cultivated for 7 years with cassava (Manihot esculenta Crantz)), maize (Zea mays) and pigeon pea (Cajanus cajan) either as sole crops or as crop combinations in various plots. After the years of cultivation, the land was naturally fallowed for 10 years. Species present in the fallow land include elephant grass (Pennisetum purpureum), guinea grass (Panicum maximum) and siam weed (Chromolaena odorata). The cultivated land is adjacent to the forestland. Various crops had been continuously cultivated on the land for over 20 years. Some of the crops cultivated during the previous years were cocoyam (Colocasia esculenta), eggplant (Solanum melongena) pepper (Capsicum sp.), sweet potato (Ipomoea batatas) and yam (Dioscorea sp.). At the time of sampling, both cassava and maize were cultivated on the land.
2.3. Soil Sampling and Chemical Analysis
- Sample collection was done at a depth of 0-20 cm using a soil auger. Twenty-one soil samples were collected from the fallow land (3 replicates in 7 plots), 3 samples were randomly collected from the adjacent forest and finally, 3 samples were also randomly collected from the adjacent cultivated land. This gave a total of 27 samples. These samples were air-dried and passed through a 2-mm sieve before analysis. Chemical analyses were carried out using standard laboratory methods. Soil pH was determined with deionized water using a soil-liquid ratio of 1: 2.5. Soil organic carbon was determined using the Walkley-Black method [15]. Organic carbon values were converted to organic matter (OM) by multiplying with the factor 1.724. Total nitrogen was determined by the modified Kjeldahl method [16]. Available phosphorus was obtained using Bray II as the extractant [17]. Exchangeable cations and cation exchange capacity (CEC) were determined following the method described by [18].
2.4. Microbial Analysis
- The number of soil microorganisms was determined using the dilution spread plate technique. Nutrient agar (NA) and potato dextrose agar (PDA) were used to culture bacteria and fungi, respectively. A dilution blank was prepared by dispersing 1 g of fresh soil in 9 ml of sterile water. This was a 10-1 dilution. After shaking, 1 ml of the dilution was transferred aseptically into another 9 ml of sterile water to give a 10-2 dilution. This process was repeated 5 times (each new dilution made from the previous dilution) until 7 dilutions were obtained (10-1 to 10-7).Dilutions 10-4 to 10-7 were inoculated on the NA plates as follows: 0.1 ml of a dilution was dropped on a plate and spread over the plate with a sterile plastic spreader. After spreading, the lid was replaced, labelled and the plate inverted for incubation which was done at 26℃ for 1 week. Each inoculated plate was in triplicate. Dilutions 10-3 to 10-5 were inoculated on the PDA plates following the same procedure. After incubation, the plates were laid out for visual observation and counting of colonies. Assuming each colony was produced by a single organism, the number of bacteria and fungi in the soil samples were calculated using the formula below:Average colony forming units (CFUs) per plate ÷ 0.1 ml ÷ dilution ÷ grams of soil per ml
2.5. Statistical Analysis
- One-way Analysis of Variance (ANOVA) was used to test for differences between the land use types. Treatment means which were significantly different were separated using Tukey’s family error rate. Correlation analysis was used to determine the interrelationship between soil chemical and microbial properties. All analyses were carried out on Minitab 17 statistical package.
3. Results and Discussion
- Soil pH of the cultivated land was significantly higher (p < 0.05) than that of the other land use types (Table 1). This is evident in the lower exchangeable acidity (H and Al) of the cultivated land compared to the other land use types. The lower pH (higher exchangeable acidity) of the forestland and fallow land may be due to their higher organic matter content, since organic acids are produced as a result of OM decomposition [19]. There was no significant difference (p > 0.05) in total nitrogen content of the different land use type, though the cultivated land had the lowest amount of this nutrient. Available phosphorus content of the cultivated land was significantly (p < 0.05) higher than that of the other land use types. Lower pH of the forest and fallow land is likely to have caused phosphorus fixation by aluminium, and iron compounds thus resulting to the reduced available phosphorus content of these land use types.
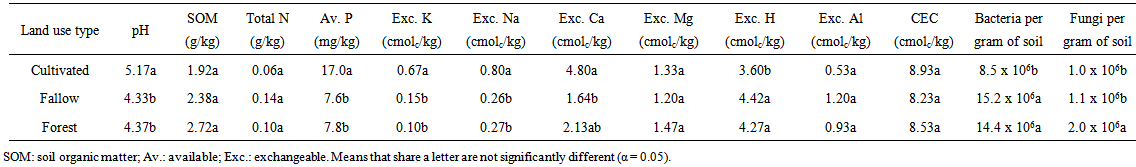 | Table 1. Chemical and microbial properties of soils from the different land use types |
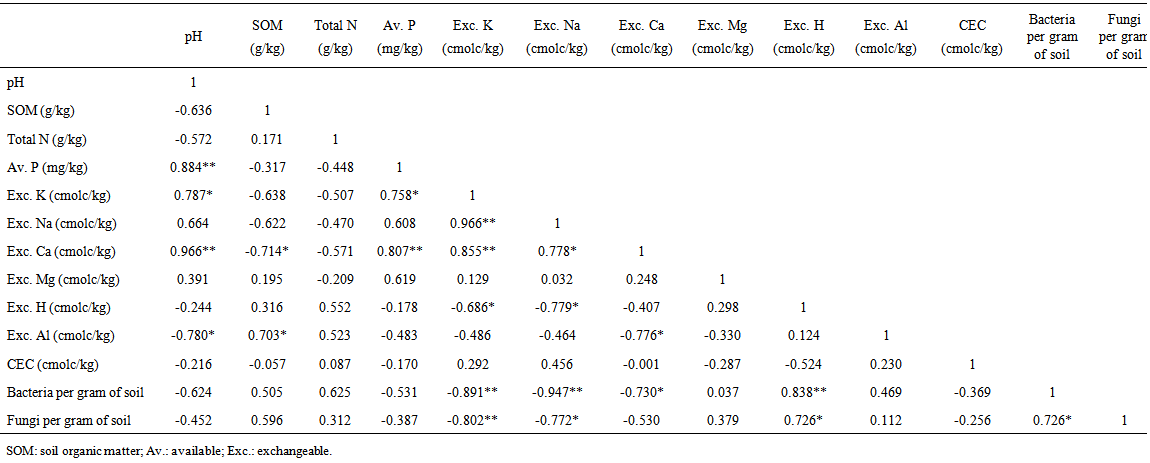 | Table 2. Correlation between soil chemical and microbial properties |
4. Summary and Conclusions
- This study carried out in southeastern Nigeria on the contribution of microorganisms to soil fertility in three land use types showed that the forestland had the highest OM content and exchangeable Mg, the fallow land had the highest total nitrogen and exchangeable acidity, while the cultivated land had the highest pH, available phosphorus, exchangeable bases (except for exchangeable Mg) and CEC. The cultivated land equally had the lowest number of microorganisms while the uncultivated land use types (forest and fallow) had greater amounts of microorganisms probably due to OM accumulation and zero tillage. It can be deduced that soil management practices and tillage operations may have reduced the contribution of microorganisms to soil fertility on the cultivated land. This means that even though the cultivated land may appear to have more nutrients compared to the forest and fallow land, these nutrients may not be sustained for a long time due to the lower number of microorganisms in this land use type. Significant correlations which existed among soil chemical and microbial properties indicate that the presence of one nutrient may affect the presence of another in the soil. These correlations equally signify the complex interrelationship that exists between soil nutrient contents and microorganisms. Using more accurate soil microbial community analysis i.e. culture-independent techniques such as phospholipid fatty acid (PLFA) analysis, nuclei acid techniques, phylogenetic analysis, fluorescent in situ hybridization (FISH) in addition to including other microbial analyses such as microbial biomass, microbial respiration and enzyme activity may improve the reliability of this experiment and thus allow for better conclusions to be drawn on the contributions of microorganisms to soil fertility.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML