-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(2): 160-165
doi:10.5923/j.ijaf.20150502.10
Changes in Crude Protein Content in the Peel and Pulp of Three Tuber Regions of Yam (Dioscorea rotundata Poir.) during Storage in Dark and Natural Light Environments
Elsie I. Hamadina1, Adeniyi O. Togun2, Mohammed K. Hamadina3
1Department of Crop & Soil Science, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria.
2Department of Environmental Biology and Crop Protection, University of Ibadan, Oyo State, Nigeria
3Biogeochem Associates Ltd, Port Harcourt, Rivers State, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop & Soil Science, University of Port Harcourt, Port Harcourt, Rivers State, Nigeria..
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Changes in crude protein (CP) content of tubers during storage in yam barns can be associated with dormancy and the start of sprouting. Storage environment is known to affect the timing of sprouting, but its effects on changes in crude protein content of yam tubers (during storage and sprouting) is not well understood. This study was conducted to determine the effects of storage environment: natural light (the yam barn) and darkness (created with jute sacs in the yam barn), on CP changes in Dioscorea rotundata Poir. tubers during storage and sprouting. This study was a 2 x 3 factorial experiment, arranged in a Randomised Complete Block design with split-split plots. Tubers of three varieties of yam (Alaako, Dodoro and Odo) were harvested prior to the onset of plant senescence, and stored in the dark and natural light environments. During storage, the tubers were sampled and partitioned into Head, Middle and Tail regions, and then each region into peel and pulp, for CP analysis. Dark storage inhibited total tuber %CP content at 4 weeks in storage (WIS). Maximum %CP content was attained in all tuber regions at 8 WIS, followed by a progressive decline sprouting. Also at 8 WIS, tuber regions showed differential response to storage environment. Head regions had higher percentage CP content under Dark storage than under Light while, Tail regions had higher % CP content under Light storage than in Dark. Furthermore, %CP content in the peel and pulp of the Middle and Tail regions was enhanced by Light storage until 8 WIS after which it was higher under Dark storage; however, the reverse was the case for the Head-peel region. The ratio of peel to pulp CP content (% per region) was 2:1 before and after 8 WIS, while it was <3:1 in either environments at 8 WIS. Dark storage delayed sprouting compared to Light storage.
Keywords: Dioscorea rotundata, Crude protein, Peel, Pulp, Storage environment, Tuber regions
Cite this paper: Elsie I. Hamadina, Adeniyi O. Togun, Mohammed K. Hamadina, Changes in Crude Protein Content in the Peel and Pulp of Three Tuber Regions of Yam (Dioscorea rotundata Poir.) during Storage in Dark and Natural Light Environments, International Journal of Agriculture and Forestry, Vol. 5 No. 2, 2015, pp. 160-165. doi: 10.5923/j.ijaf.20150502.10.
Article Outline
1. Introduction
- Dormancy is a plant physiological process that regulates the timing of sprouting of affected plant parts, and inadvertently ensures that the food quality of edible parts is maintained in storage until the following growing season. Yams exhibit long and variable tuber dormancy of ≤270 days, depending on the date of tuber initiation or the phase of dormancy at harvest ([1], [2]). Thus, the duration of dormancy declines with later tuber harvest or initiation dates so that sprouting occurs at about same time of the year ([2], [3]). Long tuber dormancy is a desirable trait for farmers and traders who wish to keep these tubers until the next planting season and/or store them for as long as possible as source of food, planting material or item of trade. On the other hand prolonged dormancy means that important genotypes could be lost from exposure to storage pests and diseases, only one planting time per year is possible, tubers are not available all year round and the pace of breeding is slow and drudgery [4]. Any success at shortening the duration of dormancy would mark a huge turn in yam research and improve tuber availability. Hence efforts to understand the mechanism of yam tuber dormancy have remained a key goal in yam research.Endogenous factors such as hormones are strongly thought to control tuber dormancy ([5-7]). Changes in protein and carbohydrate contents of tubers, as well as other physiological processes, during storage are commonly associated with the state of dormancy or its release. Based on studies at the whole tuber level and with tubers that are within the later stages of dormancy, i.e., Phase 2 to Phase 3 of dormancy [1], it is clear that the concentration of many chemical constituents change through dormancy and sprouting. As the changes occur, the nutritional value of tubers decline ([8-11]) and the tubers progress steadily into the non-dormant state ([9], [12], [13]). Crude protein, which comprises of soluble proteins, coagulate (true) protein, insoluble protein and soluble non-protein nitrogen [14], constitutes 1.0 – 8.0% of the dry matter of mature D. rotundata tubers by the end of the growing season ([15-17]). However, this value varies depending on variety, and its concentration varies vertically and horizontally across the tuber. During storage, crude protein level continues to increase until a maximum level is reached, after which it starts to decline ([9], [18]). This pattern of change suggests that some sprout inhibiting proteins are synthesised continuously, until dormancy is broken when the ratio of sprout promoting to sprout inhibiting proteins becomes high [19]. At the tuber region level, some studies on D. alata, cocoyam and D. rotundata indicate that, at harvest, crude proteins are highest in the oldest tuber region decreasing from the proximal (Head) to the distal (Tail) regions, and vertically from the peel to the pulp ([15], [20], [21]). In contrast, crude protein content has also been found to follow the order: Middle>Tail>Head [29] and Middle> Head>Tail [9]. Nonetheless, both workers reported the highest crude protein content in the Middle region. During storage, crude protein content in the three physiological regions of the yam tuber change ([9], [21]). The study of Oluoha, 1988 [9], shows that the change in crude protein content of D. rotundata follows the order: Middle > Head > Tail three months of storage. In contrast, Mozie, 1984 [21] observed the highest crude protein content in the Tail at three months after storage in a traditional yam barn, and in the Head region at five months after storage when sprouting tends to occur. Although the pattern of change of crude protein across the tuber regions appear inconsistent, which may relate to differences in the stages of tuber dormancy at the time of the studies and to the different methods of delineating the three regions, it is clear that crude protein content at the tuber regions change with time and the changes may be linked to the state of dormancy or sprouting. What is poorly understood is the effect of different storage environments on the changes in the crude protein content of the different yam tuber sections (regions, and peel/pulp), and the relationship between the changes and the commencement of sprouting. Storage environment, as influenced by its micro-climatic factors such as temperature, facilitates or suppresses the rate at which physiological processes occur [22]. Dark storage is well known to delay sprouting, which occurs most readily from the head region ([23], [24], [25]). Therefore, the objective of this study was to determine the effect of storage under natural light or darkness on the crude protein changes in peels and pulps of the three regions of D. rotundata tubers, as it relates to tuber dormancy and sprouting.
2. Materials and Methods
2.1. The Experimental Materials
- The responses of three D. rotundata varieties were studied: ‘Alaako’, ‘Dodoro’ and ‘Odo’. These varieties are commonly planted in the month of October of one year and harvested about September of the following year. The tubers were obtained from different farmers’ fields around Ibadan, Nigeria. Alaako from Alabata village, while Dodoro and Odo were from Abadina in the University of Ibadan, Ibadan. The tubers were harvested one month before the usual harvesting period by milking (removal of a large portion of the mature tuber while the foliage is still green and keeping a small portion of the head region still attached to the plant for further development into seed yam). This was done to ensure that the tubers were still developing and so, dormant.
2.2. Experimental Design and Tuber Storage
- The experimental design was Randomised Complete Block arranged as split plots. Two storage environments (natural Light and Dark conditions) formed the main plots, randomised and replicated three times. The Light environment was yam barn at the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria The barn is made up of horizontal wooden racks that are open but roofed with raffia palm leaves while the Dark environment was created by covering the horizontal wooden racks in the yam barn with brown jute sacs. The tuber regions and the peel and pulp of the regions constituted the sub and sub-sub sections plots. The experiment began in the month of August with the selection of 120 healthy tubers of various sizes for each of the three varieties used. The tubers were then placed in 12 wooden crates that were randomly and equally allocated to the two storage environments.The mean temperatures and relative humidity of the storage environments were monitored using a hygrothermograph (Figure 1 and Figure 2).
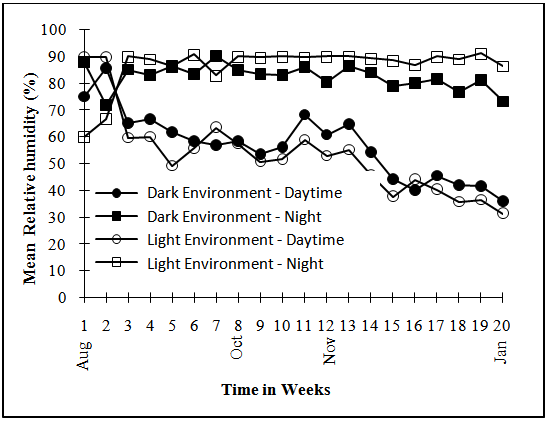 | Figure 1. Mean Day and Night Relative Humidity in Storage Environment |
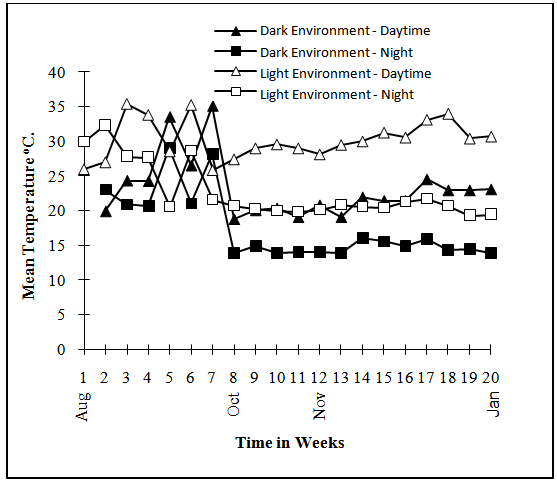 | Figure 2. Mean Day and Night Temperature in Storage Environment |
2.3. Tuber Sampling and Sample Preparation
- At the start of the experiment, and fortnightly thereafter, two tubers of each variety were randomly picked from each storage environment, washed and air-dried. Each pair of tubers was then split in halves longitudinally. One half from each tuber in the pair described above was combined to represent a tuber. Tuber halves were then divided into three regions: Head, Middle and Tail, and each were peeled to about 1.5mm. The peels from the regions were referred to as Head-peel, Middle-peel, and Tail-peel, while the respective pulps were referred to as Head-pulp, Middle-pulp, and Tail-pulp. A sample of 50g of pulp and all the peel from the respective regions were dried to constant weight at 70-75ºC. The dried samples were ground to flour (using analytical mill A-10), sieved through a 1 mm mesh size sieve and preserved in airtight packs made of polythene.
2.4. Analytical Methods
- Flour samples were analysed for total N following Kjedalh Method, as detailed by Tel and Rao (1982) [26] in the analytical laboratory at IITA Ibadan, Nigeria. Values obtained were multiplied by a factor of 6.25 to obtain percent crude protein content (%CP). Data collected were subjected to analysis of variance using Statistical Analysis System (SAS) mixed model [27].
3. Results and Discussions
3.1. Results
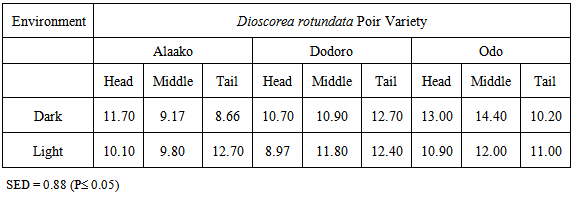
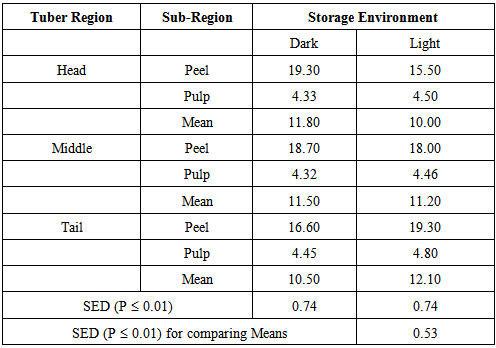
- At harvest, Tail-peel contained 9.01% crude protein (CP on dry weight basis) significantly greater than 5.25% in middle peel and 3.81% in Head-peel (P≤0.01; SED 0.42). However, the CP contents in the pulp of tuber regions were not significantly different (Table 1).
|
|
3.2. Discussions
- When D. rotundata tubers are harvested just before natural vine senescence, the Head region, which is the oldest region of the tuber and the most likely region from which sprouts emerge in intact tubers, contains lower crude protein (CP on dry weight basis) compared to the youngest, Tail region. Also, the peel contains higher crude protein than the pulp and it followed the order: Tail-peel> middle peel > Head-peel. This order is similar to that observed by Ologhobo, 1985 [12] (i.e., a gradient of Head< Tail< Middle) for D. rotundata tubers bought at Ibadan. It was however, contrary to those reported by Ferguson et al., 1980 [20] for D. alata and Mozie, 1984 [21] for D. rotundata. Although CP gradient differ among reports on D. rotundata, the range of CP content was narrow. The small differences in CP gradient could largely be due to differences in storage environment and the nearness of the tubers to the sprouting time or the lack of definite physiological limits of the morphological regions (Head, Middle and Tail) as observed for enzyme activity by Oluoha, 1988 [9].The changes in CP content observed at the tuber regions was basically a manifestation of changes in the peel section since the CP content in the pulp at the various regions was not significantly different from one another and was not significantly influenced by storage environment. Therefore, changes in CP content of peels at the various tuber regions may be better indices for studying changes in CP content over time than those of the pulp.The lower CP level observed in the tubers stored in the Dark at 4 WIS (dormancy period) suggests that dark storage leads to reduction in the rate of metabolism including rate of CP synthesis/metabolism. Storage environments that have lower storage temperature and maintain moderate to high relative humidity have long been known to slow down enzyme activity, respiration, water loss etc., and slow down the progress towards sprouting [22]. Conditions of low day/night temperatures and high relative humidity are commonly associated with dark storage conditions [28], and may contribute to the effect of dark storage on CP content. However, there were no sprouts on tubers stored in both environments at this date, thus the relationship between the CP content at this date and sprouting, could not be linked. The sharp increase in Peel CP content at the tuber regions between 4 and 8 WIS is perhaps a measure that is required for the resumption of active meristematic activity, which is known to occur later during phases 2-3 of dormancy. In many seeds, CP contents have been observed to increase during germination and decline afterward ([29], [30]). Increase in CP content is a result of new CP synthesis in growing parts and to compositional changes following degradation of other components ([29], [30]).The observations at the 8th week in storage, which was the second month after harvest and about one and -halve months away from the first visible shoot bud formation (sprouting), highlights the critical nature of this period on the progress towards sprouting. The activity of the Tail region, which is the youngest and most active region during dormancy [31], appear to be more favoured by the conditions under natural Light storage for high CP synthesis/metabolism than in the Dark. In view of the fact that: 1) the CP content of the Tail-peel under Light storage and the Head-peel under Dark storage were the same (19.3%± 0. 74); 2) CP content was higher in the Tail-peel than the Head-peel when tubers are stored in the Light environment with the reverse being the case when the tubers are stored under Dark environment; and 3) tubers stored under Light commenced shoot growth/ sprouting earlier than those under Dark storage, suggests that conditions that support high activity in the peel of the youngest region of an intact tuber may facilitate the progress of physiological processes (such as high CP synthesis) necessary for a later shoot development in the head region.
4. Conclusions
- Sprouting was first observed in tubers stored under natural Light environment, at 14 weeks in storage, while Dark storage environment delayed sprouting by two weeks. Assessing the crude protein content of the peel at the three different regions of the tuber provided useful information on the changes in crude protein content at harvest and though storage, and how storage conditions might affect change in crude protein content. The storage of tubers under light conditions support high crude protein synthesis/metabolism in the Tail-peel (the most active region of the tuber), also determined crude protein changes in the Head-peel and its subsequent dominant role in the sprouting of intact tubers.
ACKNOWLEDGEMENTS
- The authors wish to acknowledge Dr. R. Asiedu, and staff of Yam Breeding program, and Analytical laboratory at IITA, Ibadan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML