-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(2): 146-150
doi:10.5923/j.ijaf.20150502.08
In Vitro Comparative Anthelmintic Activity on Hæmonchus contortus of Two Natural Fodders (Cassia obtusifolia and Piliostigma reticulatum) Extracts Used in Burkina Faso
Wadré Saidou1, Kaboré Adama2, Bayala Balé3, Traoré Amadou2, Tamboura H. Hamidou2, Belem A. M. Gaston4
1Ministère des Ressources Animales, Ouagadougou, Burkina Faso
2Laboratoire de Biologie et Santé Animales-DPA/INERA, Ouagadougou, Burkina Faso
3Université de Ouagadougou/UFR-SVT, Ouagadougou, Burkina Faso
4Université Polytechnique de Bobo-Dioulasso/IDR, Ouagadougou, Burkina Faso
Correspondence to: Kaboré Adama, Laboratoire de Biologie et Santé Animales-DPA/INERA, Ouagadougou, Burkina Faso.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
The objective of this study was to evaluate the anthelmintic properties of aqueous extracts leaves of Cassia obtusifolia and Piliostigma reticulatum against Haemonchus contortus. For this purpose, phytochemical screening of two plants followed by eggs hatching and larval development tests subjected to five increasing concentrations (3.125, 6.25: 12.5: 25, 50 mg / ml) of aqueous extracts in comparison with a control group were conducted. We notified the presence of flavonoids, tannins and polyphenols, coumarins and alkaloids in the two extracts. All concentrations of two extracts caused a significant (P<0.05) percentage inhibition of eggs hatching and larval development of H. contortus compared to the control group. These in vitro results suggested that extracts of C. obtusifolia and P. reticulatum possess ovicidal and larvicidal properties and may be used in the control of gastrointestinal nematodes of small ruminant. However, it is suggested that in vivo tests must be conducted in order to secure rural farmers on the use of these extracts plants as natural deworming.
Keywords: Cassia obtusifolia, Piliostigma reticulatum, Antiparasitic activity, Haemonchus contortus
Cite this paper: Wadré Saidou, Kaboré Adama, Bayala Balé, Traoré Amadou, Tamboura H. Hamidou, Belem A. M. Gaston, In Vitro Comparative Anthelmintic Activity on Hæmonchus contortus of Two Natural Fodders (Cassia obtusifolia and Piliostigma reticulatum) Extracts Used in Burkina Faso, International Journal of Agriculture and Forestry, Vol. 5 No. 2, 2015, pp. 146-150. doi: 10.5923/j.ijaf.20150502.08.
Article Outline
1. Introduction
- In Burkina Faso, national ruminant herd is numerically important and composed of 8 566 448 cattle, 8 490 513 sheep and 12 712 705 goats [1]. These animals and particularly small ruminants constitute an important factor in food and nutritional security of the population and also a fight against poverty by increasing the incomes of farmers in the order of 3% per year [2]. They represent a source of sure savings and a checking account easily converted into cash for farmers. However, their raising is still confronted with many development constraints which paralyze ruminant productivity [2]. Indeed, dominant raising system in Burkina Faso is extensive where malnutrition and gastrointestinal parasitism were permanent and cause low performances of animals [3, 4]. This situation led rural farmers with low-income to seek alternative solutions through the use of natural fodder plants with anthelmintic effect as Cassia obtusifolia [5] and Piliostigma reticulatum [6, 7] to control gastrointestinal parasitism.Formerly called Cassia tora L., C. obtusifolia is of the Caesalpiniaceae family and native to tropical Africa and India. This is an annual woody herbaceous shrub that can grow to a height of 2.5 m and presents alternate, composed and paripinnate leaves. According to Farah et al. [5], the plant has several pharmacological activities in modern and traditional medicine.P. reticulatum belongs to the family of Caesalpiniaceae. It is a shrub with rounded and bushy tops [8] that is encountered in Sahel and Sudan-sahel regions. These leaves are composed of two lobes and are usually simple, alternate and green matt gray [9]. In herbal medicine, P. reticulatum is involved in the treatment of many diseases [10, 11]. In this context, the objective of this study was to evaluate the potential in vitro anthelmintic effects of leaves extract of these two plants against eggs and infective larvae of parasitic nematode Haemonchus contortus of sheep.
2. Materials and Methods
2.1. Plant Material and Preparation of Extracts
- Leaves of C. obtusifolia and P. reticulatum were harvested at Saria station of “Institut de l’Environnement et de Recherches Agricoles” in Burkina Faso. The specimens of two plants were then identified from the herbarium of the “Centre National de la Recherche Scientifique et Technologique” in Ouagadougou.Then, harvested leaves were washed with water and dried for a week before being pulverized to achieve the maceration according to the procedure of traditional healers in the country. To this end, 25 g of powder of each plant were prepared in 500 ml of distilled water for 24 h. Macerated extracts were filtered over cotton wool and filter paper and concentrated by freeze dryer at 50°C for 4-5 days before being stored at refrigeration (at 4°C) for in vitro tests. One quarter of hour before the tests, these obtained extracts were dissolved in phosphate buffered saline (PBS pH: 7.2) to obtain six increasing concentrations (3.125 - 50 mg/ml) and used immediately. In addition, a part of each plant’s powder was used for the characterization and phytochemical.
2.2. Animal Material
- Stomachs of sheep naturally infected were collected in refrigerated slaughterhouse in Ouagadougou and used to collect adult of the H. contortus parasite. Then, H. contortus eggs were recovery using method previously described by Jabbar et al. [12] for in vitro tests.
2.3. Phytochemical Analysis
- Powders of the two plants were subjected to phytochemical screening to identify key groups of secondary metabolites including alkaloids, flavonoids, tannins and polyphenols, saponins, coumarins and glycosides anthracenesusing standard procedures described by Ciulei [13].
2.4. In Vitro Tests Methodology
- For the tests, inhibition egg hatch and larval development tests were applied and adapted from Jackson and Hoste [14].
2.4.1. Inhibition of Egg Hatch
- The inhibition of egg hatch test was performed using approximately 100 eggs per 1 ml in Nunc cryotube (3.6 ml). After that, 1 ml of each extract of two plants (C.obtusifolia and P. reticulatum) at different concentrations (3.125, 6.25, 12.5, 25 and 50 mg / ml) prepared with PBS was added in each Nunc cryotube and PBS only as control group. Three replicates for each concentration of extract and control were performed. All Nunc cryotube were incubated under humidified condition at ambient temperature (27°C) for 48 h. Then, three drops of formalin solution (10%) was added to each Nunc cryotube to stop further hatching and the percentages of inhibition and larvae L1 were counted under an inverted microscope.
2.4.2. Inhibition of Larval Development
- The test was conducted using approximately 100 eggs in 1 ml in Nunc cryotube (3.6 ml) and subjected to incubation at room temperature for 48 h in the obscurity to obtain egg development in larvae of first stage (L1). Then, 200 µl of nutrient Agar (DIFCO: 2%) were added into each Nunc cryotube before being subjected to the same treatments of the previous test with the extracts of both plants and control. Three repetitions were performed. All tubes were further incubated for 7 days before three drops of formalin solution (10%) were used to stop all development in Nunc cryotube. Futher, all infective larvae obtained (L3) was determined microscopically.
2.5. Statistical Analyses
- Results of the phytochemical analysis of the two plants tested were noted - (negative) or + (positive). Data from egg hatching and larval development inhibition tests were transformed to log (x + 1) before being submitted to one-way analysis of variance. The comparison of means values obtained (expressed as mean ± standard deviation) was performed using Tukey-Kramer test. Kruskal-Wallis test was used to evaluate the effect of extracts. All analysis was performed with Costat software (version 6.20.4) at 0.05.
3. Results
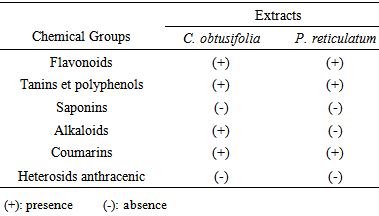
3.1. Phytochemical Composition of Plant Extracts
- Table 1 presents the result of phytochemical analysis of both two plants. Leaves of C. obtusifolia contain more chemicals than those of P. reticulatum. In all plants, applied analysis revealed the presence of flavonoids, tannins and polyphenolsand coumarins.
|
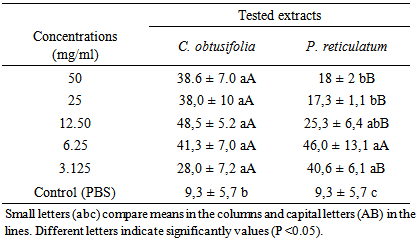
3.2. Inhibition of Hatching Eggs
- The percentage of inhibition of H. contortus hatching eggs observed by the concentrations of both extract plants and control is presented in Figure 1 and Table 2. The percentage of inhibition achieved with control was 9.3% and those of plant extracts ranged from 28% to 48.5% and 17.3% to 46% for C. obtusifolia and P. reticulatum, respectively. The percentage of inhibition of control was significantly (P < 0.05) lower compared to those presented by all concentrations of two plant extracts. Between the extracts, only the concentration of 6.25 mg/ml did not show a significant difference (P <0.05).
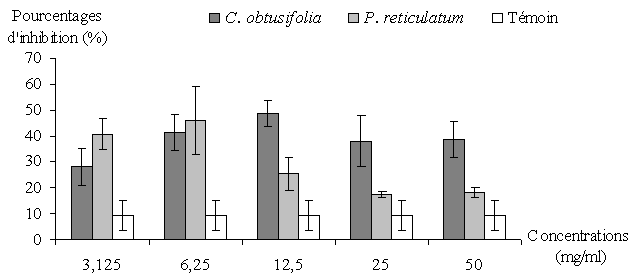 | Figure 1. Response profile of egg hatching of H. contortus submitted at the concentrations of C. obtusifolia and P. reticulatum compared to the control |
|
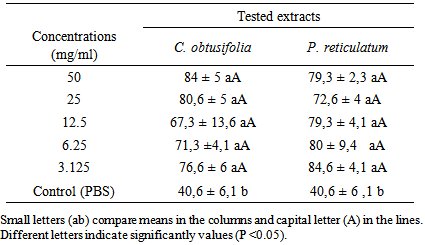
3.3. Inhibition of Larval Development
- Table 3 and Figure 2 present the percentage of inhibition of H. contortus larval development obtained with the concentrations of extract plants and control. The percentage of inhibition of control (PBS) was 40.6%, while those of the extracts ranged from 67.3% to 84% for C. obtusifolia and 72.6% to 84.6% for P. reticulatum. The percentage of inhibition of control was significantly (P <0.05) lower compared to those of the extract concentrations plant of two plants between which no significant difference (P <0.05) were observed. At the end of the test, all developped eggs to the stage LI and L2 died in each plant extract.
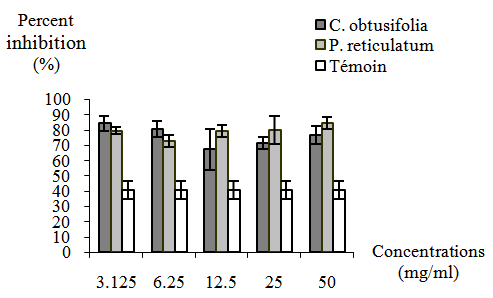 | Figure 2. Response profile of larval development of H. contortus submitted at the concentrations of C. obtusifolia and P. reticulatum compared to the control |
|
4. Discussion
- The main aims of this study were to determine the ovicidal and larvicidal effects of C. obtusifolia and P. reticulatum on H. contortus which is commonly used as animal model to evaluate the anthelmintic activity of medicinal plants [12, 15]. In addition, it is one of the most dominant digestive parasites in Burkina Faso [16, 17] where the anthelmintic activity of C. obtusifolia and P. reticulatum was not studied. Also, these plants are present throughout the whole country and are natural fodders that are little used as ruminant food notably in the extensive raising which is dominant in the country [1].In the study, the obtained results during the two tests show that all the concentrations of two plant extracts inhibit egg hatching and larval development of H. contortus compared to control. In addition, eggs and inhibited larvae died, denoting the ovicidal and larvicidal activity of the two extracts. Similar studies have reported the effectiveness of plant extracts such as Spigelia anthlemia on H. contortus by Assis et al. [18] and Chenopodium ambrosioides on the parasitic nematode Heligmosomoides bakeri by Wabo Pone et al. [19].The anthelmintic activity observed during the study may be due to the presence of secondary metabolites contained in the two plant extracts that would act synergistically or individually on the eggs and larvae of H. contortus. Indeed, our studied extracts contain secondary metabolites as flavonoids, tannins and polyphenols, coumarins and alkaloids which contain various pharmacological properties that are reported by Cowan [20] and several authors. According to Wabo Pone et al. [21], basicity condition created by alkaloids is detrimental to the survival of larvae that prefer acidic conditions. The works of Lee et al. [22] on C. elegans and Reddy and Seetharam [23] on Pheretima posthuma report that flavonoids have anthelmintic activity. For tannins, including condensed tannins, their anthelmintic activities have been mentioned by Hoste et al. [24]. In all cases, active secondary metabolites observed in the two extracts probably penetrated the egg shell and the cuticle of larvae by osmosis through the circulatory system to stop the development process of segmentation of blastomeres and larvae and then cause death by starvation [25] or paralysis [26]. In the study, the inhibition of larval development is due especially to the L1 larval mortality in the egg shell. In addition, the comparative analysis of the relative percent inhibition of tested concentrations shows that low doses (3.125mg/ml) of P. reticulatum extract is more ovicidal and larvicidal than C. obtusifolia extract although it would contain less secondary metabolites. Similar deductions on the mechanisms of anthelmintic activity of medicinal plants were made with Chenopodium ambrosioides and Tithonia diversifolia extracts on the parasitic nematode Heligmosomoides bakeri by Wabo Poné et al. [19] and Leuceana leucocephala and Gliricidia sepium extracts by Kaboré et al. [27]. To conclude, the results from this study showed that the extracts from C. obtusifolia and P. reticulatum have shown active in vitro anthelmintic effect against eggs and larvae of H. contortus. These anthelmintic efficacies could justify their use against gastrointestinal parasites of small rural ruminants by smallholders and pastoralists in farming areas. However, further in vivo toxicological and parasitological studies may be done because results obtained from in vitro conditions could be different from in vivo conditions [28].
ACKNOWLEDGEMENTS
- Authors acknowledge PAES / UEMOA (VPMAP: P Z1 - IAD - 002) and IAEA-BKF 5014 projects for the support provided to the study implementation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML