-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2015; 5(2): 131-137
doi:10.5923/j.ijaf.20150502.06
Assessment of Drought Tolerant Barley Varieties under Water Stress
Maisa’a Farah Haddadin
National Center for Agricultural Research and Extension, Al-Baq’a, Amman, Jordan
Correspondence to: Maisa’a Farah Haddadin, National Center for Agricultural Research and Extension, Al-Baq’a, Amman, Jordan.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
Four barley varieties (Rum, Athroh, and Muta’a) including a Jordanian barley landrace were subjected to assess their drought tolerance in three water stress regimes. The water stress regimes are T1 - 75% of available water served as the control (non-stress), T2 - 25 % of available water after emergence served as Sustain water stress, and T3 - 25 % of available water at booting stage served as late water stress respectively. The objective of this study was to identify drought tolerant varieties by using drought susceptibility index (DSI) under different water stress treatments to set recommendations on their possible use in drought tolerance breeding programs. Results showed that water stress reduced grain yield and its components. Sustained water stress (T2) exhibited the highest reductions in grain yield and its components. Number of spikes plant-1 and number of kernels spike-1 were more sensitive to water stress than 100-kerrnel weight. From the concept of drought susceptibility index DSI (less grain yield losses), local landrace was the most tolerance variety because it exhibited low grain yield losses under water stress treatments. Accordingly it could be considered as a potential source of genes for drought tolerance in plant breeding.
Keywords: Barley, Water stress, Landrace, Drought susceptibility index (DSI), Grain yield
Cite this paper: Maisa’a Farah Haddadin, Assessment of Drought Tolerant Barley Varieties under Water Stress, International Journal of Agriculture and Forestry, Vol. 5 No. 2, 2015, pp. 131-137. doi: 10.5923/j.ijaf.20150502.06.
Article Outline
1. Introduction
- Barley (Hordeum vulgare L.) is the first important winter cereal crop grown in arid and semi-arid regions in West Asia and North Africa (WANA), where terminal drought stress prevalent during grain filling period [1]. Kilic et al., [2] reported hat due to earliness and its ability to escape terminal drought-stress, barley would be a suitable crop in areas where irrigation is poorly available. Low yields are partly caused by the unavailability of good yielding cultivars with stress tolerance. Drought stress as an abiotic stress is one of the most common environmental stresses that affects growth and development of plants [3]. Loss of yield is the main concern of plant breeders, and hence emphasize on yield performance under stress conditions [4]. Drought affects morphological, physiological, biochemical and molecular processes in plants resulting in growth inhibition. The extent of these changes is dependent on the time, stage and severity of environmental stress [5]. Drought stress, caused by low and erratic rainfall, is one of the major constraints for barley production in WANA region [1]. Low grain yield and even crop failure are common in this region, where locally adapted landrace populations of barley are predominantly cultivated [6]. Barley landraces are the oldest domesticated plants in history (10,000 years ago in the Fertile Crescent), genetically heterogeneous populations and it had good adaptation to local environmental conditions [7]. Climate changes will increase the frequency of droughts, while drought is a global issue, its effects need to be addressed locally because every dry area has its own type of drought [8]. Global warming poses new challenges to plant breeding. In many places, current varieties will no longer be suited for cultivation, moreover, breeding for drought resistance is complicated by the lack of fast, reproducible screening techniques and the inability to routinely create defined and repeatable water stress conditions when a large amount of genotypes are to be evaluated efficiently [9]. The relative yield performance of genotypes in drought stressed and more favourable environments seems to be common starting point in the identification of traits related to drought tolerance and the selection of genotypes for use in breeding for dry environments [10]. According to Fernandez [11], genotypes can be divided into four groups based on their yield response to stress conditions: (1) genotypes producing high yield under both water stress and non- stress conditions (group A), (2) genotypes with high yield under non-stress (group B) or (3) stress (group C) conditions and (4) genotypes with poor performance under both stress and non-stress conditions (group D). There is general agreement that modern high yielding varieties are more adapted to favourable growing conditions, while old varieties and landraces have more stable yield under drought stress conditions [12, 13]. Ceccarelli et al., [6] claimed that the most effective way to improve the productivity of crops grown in less-favourable areas is to use locally adapted germplasm and select form target environments. Conventional breeding and high yielding varieties have had virtually no success in these less-favourable areas. But this lack of success has had a positive effect in preserving biodiversity because in these environments all the barley grown is landraces, which have evolved directly from the wild progenitor in hostile environments providing a rich reservoir of genes for adaptation and survival to the harsh natural environment [14]. Achieving a genetic increase in yield under harsh environments has been recognized to be a difficult challenge for plant breeders while progress in yield has been much higher in favourable environments [15]. Thus, drought indices which provide a measure of drought based on yield loss under drought conditions in comparison to normal conditions have been used for screening drought-tolerant genotypes [16]. Selecting different genotypes under environmental stress conditions is one of the main tasks of plant breeders for exploiting the genetic variations to improve the stress tolerant cultivars [17, 18]. Agronomic traits such as grain yield and its components are the major selection criteria for evaluating drought tolerance under field conditions [18, 19].Using useful stress indices is one of the most important tools for plant breeders to seek for high tolerance genotypes [20, 21]. A drought susceptibility index (DSI), which provides a measure of stress tolerance based on minimization of yield loss under stress, as compared to optimum condition, rather than on yield stress per se which has been used to characterize relative drought tolerance [22, 23]. Its values were used for differentiating the overall stress tolerance of genotypes [24]. The DSI index was highly negatively correlated with grain yield at the driest sites, indicating that larger yields were associated with higher levels of drought tolerance or with high stability [23, 25]. Genotypes identified as stress tolerance using DSI values should possess tolerant mechanism which may need to be incorporated into germplasm with higher yield potential for the development of high yielding and stress tolerant varieties [24]. The objective of the study was to identify drought tolerant varieties by using drought susceptibility index (DSI) under different water stresses to set recommendations on their possible use in drought tolerance breeding programs.
2. Materials and Methods
2.1. Plant Material
- Three Jordanian certified barley varieties were obtained from the National breeding programme in National Center for Agricultural Research and Extension (NCARE), namely: Rum, Athroh, and Muta’a besides one Jordanian barley Landrace (Arabi Abiad) as a local check where Rum and Athroh are six-rows barley, while the other varieties are two-rows.
2.2. Pot Experiment
- The experiment was conducted at Rabba station (Elevation 920 m, Longitude 35° 45´ and Latitude 31º 16´) belongs to NCARE, in an open greenhouse used as rainout shelter house in winter and for protection from bird attacks at grain filling period. Plastic pots, 26cm in diameter and 40cm deep were filled with 8 kg soil taken from a field at Rabba station which left fallow for many years. The soil texture class is clay-loam, pH = 8.0, total N% = 9.4 and available P = 58.0 ppm.On 1st January 2011 barley seeds were sown at the rate of eight seeds per pot. The available soil moisture was determined by difference between the soil water content at field capacity and the permanent wilting point (PWP at -15 bars). Field capacity was estimated as the difference obtained between soils saturated with water (soil weight after drainage was stopped at - 0.33 bars) and oven dry weight of saturated samples after soil being dried at 105 °C for 24 h.The permanent wilting point was estimated by subtracting oven dry weight from soil samples subjected to - 15 bars. Soil moisture content was measured using the gravimetric method. Soil samples were weighed before and after oven drying at 105 °C for 24 h and the weights divided by the weight of the oven-dry soil.All pots were irrigated to 75 % of available water from seeding date until emergence and then seedlings were thinned to five healthy plants per pot when the second leave stage expanded. Plants were subjected to three water stress regimes: 75% of available water served as the control (non-stress) (T1), 25 % of available water after emergence served as sustain water stress (T2) and 25 % of available water at booting stage served as late water stress (T3), respectively. Pots were irrigated once every other day and were kept free of weeds by continuous hand weeding. The moisture status was determined gravimetrically by weighing and watering the pots. Irrigation continued until 24th April, after that irrigation withholds on all pots until harvesting. The experiment was arranged as a split-plot in a completely randomized block design with three replications. The water stress treatments were randomly assigned to main plots and the varieties were randomly assigned to subplot. Phonological and morphological characteristics viz., plant height (cm), days to heading (day), days to maturity (day), and grain filling duration were measured during plant growth. Grain yield and yield components such as biological yield (g plant-1), straw yield (g plant-1), number of spikes plant-1, number of kernels spike-1, 100-kernels weight (g), and harvest index (%) were recorded at the end of life cycle.
2.3. Estimation of Drought Susceptibility Index (DSI)
- A drought susceptibly index (DSI) for grain yield and its components was calculated using the following formula [22]:
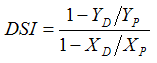 Where, YD = mean yield of individual genotype under stress condition YP = mean yield of individual genotype under control conditionXD = mean of all genotypes under stress condition XP = mean of all genotypes under control condition
Where, YD = mean yield of individual genotype under stress condition YP = mean yield of individual genotype under control conditionXD = mean of all genotypes under stress condition XP = mean of all genotypes under control condition 2.4. Statistical Analysis
- Analysis of variance (ANOVA) was used to test genotype and water stress treatment effects and their interaction. Data was analyzed using two-way ANOVA using the statistical package MSTAT-C (Michigan State University, East Lansing, MI, and USA) statistical software. The differences between the means were compared using least significant differences at P < 0.05. Mean separation was performed using least significant differences (LSD) at 5% probability.
3. Results
3.1. Agronomic Traits
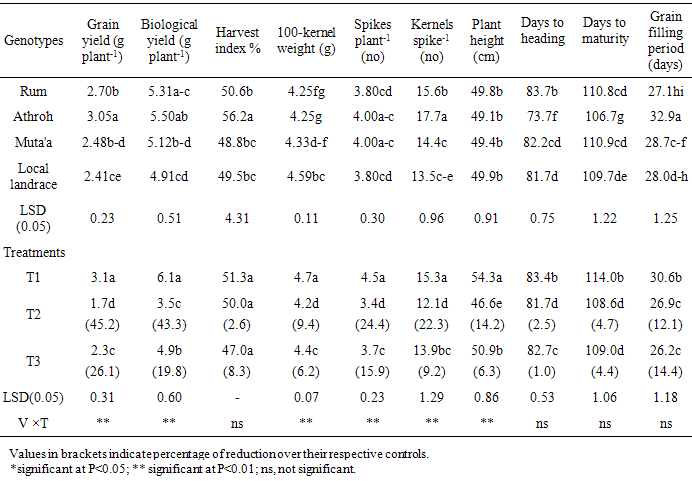
- Highly significant (P<0.01) interactions between variety × treatment (V × T) were recorded for grain yield (g plant-1), biological yield (g plant-1), 100 - kernel weight (g), spikes number plant-1, number of kernels spike-1 and plant height (cm) (Table 1). The results showed wide range of variation for all traits recorded in this study. For example, the average grain yield ranged from 2.41 g plant-1 in Local Landrace to 3.05 g plant-1 in Athroh.
|
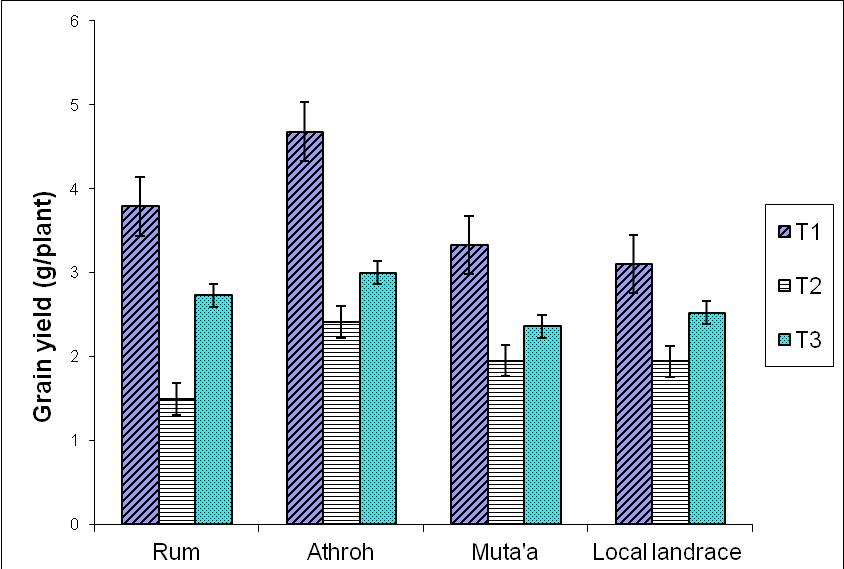 | Figure 1. Interactive effects of varieties and water stress treatment on grain yield  |
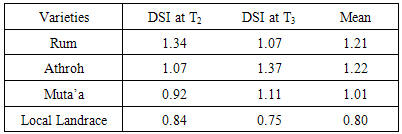
3.2. Drought Susceptibility Index for Grain Yield (DSI)
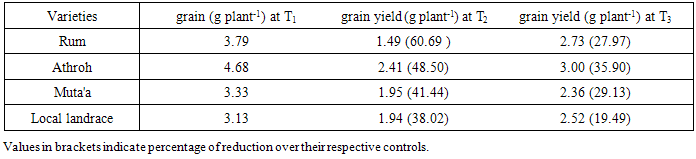
- The mean DSI values based on grain yield ranged from 0.84 to 1.34, and from 0.75 to 1.37 in response to continuous water stress (T2), late water stress (T3), respectively (Table 2). The most tolerant variety under different water stress treatments was the Local Landrace. DSI values under (T2) and (T3) were 0.84, and 0.75 in the local landrace, respectively. However, the least tolerant varieties were Athroh followed by Rum with DSI values ranging from 1.07 to 1.37, and from 1.34 to 1.07 under T2, and T3, respectively.
|
|
4. Discussion
- Water stress treatments significantly (P = 0.01) reduced yield and yield components, in addition reduced phonological traits (shortened days to heading and maturity) among the four barley varieties. These results confirmed the earlier findings of Shakhatreh et al., [25], Samarah [26] and Samarah et al., [27] consistent with Al-Rjoub [28] and Abdel-Ghani [29]. The grain yield significantly decreased when drought stress treatments were imposed as compared with the non-stress treatment (control). Yield reduction was more prominent under continuous water stress (T2) (Figure 1). Athroh the earliest headed cultivar had the highest mean grain yield among water stress treatments although it had high grain yield losses in agreement with [25, 26, 30]. The results indicated that earlier heading in barley is the better adaptation to low rainfall areas where terminal drought conditions are very likely to prevail. More over they emphasized that barley varieties which had higher grain filling rate, shorter grain filling period, and better grain yield might be able to avoid terminal drought stress in semiarid area. Rizza et al., [31] assigned that selection for high grain yield under favourable conditions could produce genotypes suitable in both stress and non-stress environment. However, in WANA region, rainfall is usually limited at the end of the growth cycle and therefore grain filling stage is commonly the most affected by water stress. This will cause a severe reduction in grain yield and might also lead to crop failure in seasons combined with severe terminal drought [27, 32]. Selection of genotypes adapting to terminal drought stress is therefore a priority in WANA. In this study, a high level of variability was detected among tested barley varieties when the water stress was imposed during grain filling. Under late water stress T3, the least tolerant cultivar was Athroh with 35.9% grain yield reduction. However, the most tolerant local landrace with 19.49% grain yield reductions.On the other hand, from the concept of DSI (less grain yield losses), Local Landrace was the most tolerance cultivar because it exhibited low grain yield losses under water stress treatments (Table 2), Although it had low grain yield under none-stress treatment (T1). These results were in agreements with Mun˜oz et al., [33] and Voltas et al., [34] who indicated that landraces performed better under stress conditions while old and modern genotypes performed better under optimum conditions. Moreover, Pswarayi et al., [35] emphasized that not all landraces yielded better than modern genotypes in low yielding stress sites but only those originating from the stress environments. Thus it may not be true to generalize that landraces are better than modern genotypes but to qualify the generalization and say better within specific environments of origin. Fayaz and Arzani [19] reported cultivars with low DSI values are drought tolerance because they have lesser reduction in grain yield under stress compared with non-stress condition. Moreover, Haddadin et al., [36] indicated that genotypes with low DSI values might represent a valuable genetic resource for enlarging the genetic variation of barley breeding programs for drought tolerance.All yield components were significantly contributed in grain yield losses. The reduction in yield and yield components in barley in response to late and continuous water stress was previously reported by [26, 27, 32, 37]. In this experiment the grain filling period was shortened (3 to 4 days) by imposing late drought stress which might be cause of grain yield reductions. These results in agreement with, Samarah [26] and Samarah et al., [27] who relieved that post anthesis drought stress shortened grain filling period and reduced the grain yield regardless of the stress severity. Likewise, Moustafa et al., [38] confirmed that mid and late season drought stress shortened the grain filling period by 10 to 11 days. Number of spikes plant-1 and number of kernel spike-1 were more sensitive to drought than 100-kerrnel weight. These results in agreement with the findings of other researchers [27, 29, 39] who found that number of kernels-1 spike and spikes m-2 were the most yield components sensitive to drought while kernel weight remains relatively stable due to high remobilization of stored preanthesis assimilates to grains. Moreover, Samarah [26] and Sanchez et al., [40] attributed the reduction in number of spike plant-1 under drought due to the increase in the number of sterile spike plant-1 and the decrease in number of fertile spike plant-1 .The reduction in total grain yield under the drought stress treatments could be due to the reduction in grain yield components such as individual grain weight [37, 41], grain number per spike [26, 32, 36, 42] and spike number per square meter [32, 42] and/ or to the reduction in number of tillers per plant. The reduction in grain yield was attributed to the decrease in number of fertile grain and grain number per spike and the increase in the tiller number bearing sterile spikes and grains [26, 40]. Moreover Farshadfar et al., [43] and Poursiahbidi et al., [44] indicated that a significant and negative correlation coefficient was found between grain yield-1 plant and number of grain-1 spike under stress condition.
5. Conclusions
- This study revealed that under water stress condition, cultivars with low DSI values are drought tolerance because they have less reduction in grain yield. As the local landrace was better adapted to water stress treatments, it is recommend to be used as parents for improvement of drought tolerance in other cultivars.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML