-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(6): 435-439
doi:10.5923/j.ijaf.20140406.03
Development of SCAR Molecular Markers in Eucalyptus saligna and Eucalyptus tereticornis
Kettener K.1, Fuchs M. C. P.1, Madacki A. C. A.1, Gonzales E.2, Souza I. C. G.3, Oda S.3, Marino C. L.1
1Department of Genetics, Institute of Biosciences, Botucatu, São Paulo, Brazil
2Futuragene, Itapetininga, São Paulo, Brazil
3Suzano Pulp and Paper, Itapetininga, São Paulo, Brazil
Correspondence to: Kettener K., Department of Genetics, Institute of Biosciences, Botucatu, São Paulo, Brazil.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The genus Eucalyptus includes over 700 species, some of which are the most widely planted hardwoods worldwide. Each species of Eucalyptus present different characteristics regarding its wood quality and yield. This fact makes it very important to work with known species to optimize handling and conservation of forest resources. Some of them are morphologically similar, making it difficult to differentiate by simple observation. An alternative approach is to develop molecular methods for the species differentiation. Using a Bulk Segregant Analysis (BSA) with 59 RAPD (Random-Amplified Polymorphic DNA) primers of Operon Technologies Inc. Kits, polymorphic DNA fragments between Eucalyptus species were isolated and SCAR (Sequence Characterized Amplified Regions) markers designed for Eucalyptus saligna and Eucalyptus tereticornis.
Keywords: Bulk Segregant Analysis, RAPD, Myrtaceae, SCAR
Cite this paper: Kettener K., Fuchs M. C. P., Madacki A. C. A., Gonzales E., Souza I. C. G., Oda S., Marino C. L., Development of SCAR Molecular Markers in Eucalyptus saligna and Eucalyptus tereticornis, International Journal of Agriculture and Forestry, Vol. 4 No. 6, 2014, pp. 435-439. doi: 10.5923/j.ijaf.20140406.03.
Article Outline
1. Introduction
- Eucalyptus genus is an important forest culture in the world economy, due to certain characters that confer advantages, both in their introduction and maintenance in different regions [1]. The genus presents wide species-diversity, with many varieties and hybrids (more than 900) [2, 4].Eucalypts are native to Australia and north islands, occurring from the tropics to latitude 43° south [5]. The latest taxonomic revision [3] of the eucalypts recognizes over 700 species that belong to 13 main evolutionary lineages. Most species belong to the subgenus Symphyomyrtus, and it is mainly species from three sections of this subgenus that are used in plantation forestry such as Eucalyptus grandis and Eucalyptus urophylla (section Transversaria), Eucalyptus globulus (section Maidenaria), Eucalyptus camaldulensis (section Exsertaria), Eucalyptus saligna (section Latoangulatae) and Eucalyptus tereticornis. E. saligna, native to the southern coast of New South Wales and south Queensland, Australia, is to be found in a region where frost is frequent (more than 60% of the year), which justifies its wide use in breeding programs aimed at cloning frost-resistant species, with the specific characteristics of increased growth and density in cold regions [1, 7]. The natural occurrence of E. tereticornis in Papua New Guinea and Australia (Victoria, New South Wales and Queensland), regions with dry periods of up to seven months during the year, explains its importance in drought-tolerant-clone development [1]. Besides drought tolerance, other outstanding characteristics are high disease-resistance potential and wood density [7].Each species of Eucalyptus present different characteristics regarding its wood quality and yield. This fact makes it very important to work with known species to optimize handling and conservation of forest resources. There are two moments in which the correct identification between these species is very important and cannot be performed by visual methods: in the nursery, as a seedling before planting it, and in debarked wood.The precise classification requires tools comprising specific molecular-biology techniques [4], applicable to morphological identification. The development of species-specific molecular markers becomes a feasible alternative in solving this conflict accurately, quickly and at low cost.Forestry companies, by using species-specific molecular markers to determine matrices and launch authentic hybrids [9-12], manage to avoid taxa introgression, with the possible aftermath of negative consequences, such as variability-loss and genetic-assimilation. ISSR (Inter-Simple Sequence Repeat) molecular markers have already been developed for E. urophylla, E. grandis and E. camaldulensis species [13]. The RAPD (Random-Amplified Polymorphic DNA) analysis has become a method for estimating genetic diversity in plant populations or cultivars [14, 15]; it was also used by Paran and Michelmore to develop a technique known as sequence characterized amplified regions (SCAR) [16]. We have used this technology to develop SCAR markers for E. saligna and E. tereticornis to quick differentiation of these two species. These specific primers lead to positive or negative amplification in target-containing and non-target - containing samples, respectively; they also can be used to generate amplification products of different sizes in closely related samples.
2. Materials and Methods
2.1. Plant Material
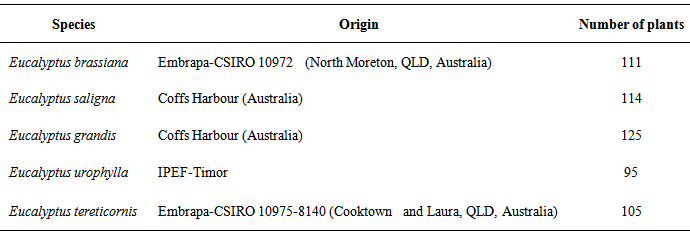
- SCAR marker development was carried out in two groups of four-month-old Eucalyptus sp. seedlings (Table 1), supplied by Suzano Pulp and Paper breeding program.
|
2.2. DNA extraction
- DNA extraction was based on [23] protocol, with a reduction in CTAB concentration of 10% to 5%, and by using twice volume. Extracted DNA was quantified by a spectrophotometer and comparison of band intensities with known standards of GeneRuler 1 KB DNA Ladder (Thermo Fisher Scientific, GA, USA) on 0.8 % agarose gels. Each DNA concentration was adjusted to 20 ng/μl in sterile miliQ water and stored at -20 °C.
2.3. BSA (Bulk-Segregant Analysis) and RAPD (Random-Amplified Polymorphic DNA)
- Bulk Segregant Analysis (BSA) Technique [17] was used to identify RAPD Markers. Two separate DNA bulks were prepared, each containing an equal amount of DNA (500ng) from ten individuals for each eucalyptus species, in a final concentration of 50ng/μl. Each bulk was identified with the first letter of the species epithet. A total of 59 RAPD primers (Operon Technologies Inc.) were screened between the pools. RAPD reactions were performed in a 96-well thermal cycler (MJ Research - PTC 100), with one step at 96°C for 3 min and 41 cycles at 92°C for 1 min, 35°C for 1 min, and 72°C for 2 min and 30s, followed by one step at 72°C for 10 min. Following 1.5% agarose gel electrophoresis and ethidium bromide staining, amplified patterns were visualized over a UV transilluminator and photographed by a digital camera. RAPD amplified bands were scored visually according to their presence or absence for the species studied. Only clear, unambiguous and reproducible RAPD molecular markers were taken into account. The reproducibility of each scored marker was checked by two RAPD experiments.
2.4. SCAR Marker Development
- DNA fragments of selected RAPD markers were isolated from agarose gel with a Gel Band Purification kit (Amersham), as recommended by the manufacturers. Purified fragments were cloned into a pGEM-T Easy Vector System I vector (Promega), and then inserted into competent cells of a DH5α-FT UltraMax strain (Life Technlogies, GibcoBRL), as recommended by the manufacturers.DNA sequencing reactions were obtained using 400 to 500 ng of plasmid DNA with forward and reverse M13 primers (1 mM), according to the protocol of ABI Big Dye Terminator Version 3.1 Cycle Sequencing kit (Applied Biosystems). Amplification conditions in a thermocycler (MJ Research – PTC 100) were an initial 2 minutes at 96°C, followed by 40 cycles, each of 30 seconds at 96°C, 30 seconds at 55°C and a final 4 minutes at 60°C. The reaction was purified by adding 80 uL of 75% isopropanol, incubated for 15 minutes at room temperature, and then centrifuged for 45 minutes at 4000 rpm. The supernatant was discarded, and 1ml of 70% ethanol subsequently added for washing. Centrifugation was repeated for 10 minutes at 4000 rpm, whereupon the supernatant was again discarded and the pellet dried at room temperature. After resuspension in 10μL of formamide, the pellet was sequenced on an automated ABI / Hitachi 3100 Genetic Analyzer sequencer (Applied Biosystems).Nucleotide sequence data were compared against the GenBank nucleotide sequence database (BLAST search) and the Phytozome database (http://www.phytozome.net). They were then analyzed with the Primer3 [18] software in order to design pairs of PCR primers (approximately 20-mers) to obtain SCAR molecular markers characteristic of E. saligna and E. tereticornis. Oligonucleotides were synthesized by Sigma-Aldrich. PCR conditions for the amplification of SCAR molecular markers were prepared in a final volume of 25 μl containing 150 ng DNA, 1x PCR buffer, 1.92 mM MgCl2; 1μg/μL of Bovine Serum Albumin, 0.8 mM dNTPs, 15 ng / primer (Operon Technologies Inc.) and 1U Taq DNA polymerase (Invitrogen - Life Technologies). The final reaction volume was completed with 13 μL of autoclaved di-ionized water. All amplifications were repeated at least in three independent experiments.
3. Results and Discussion
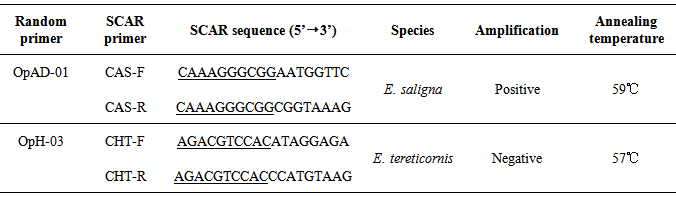
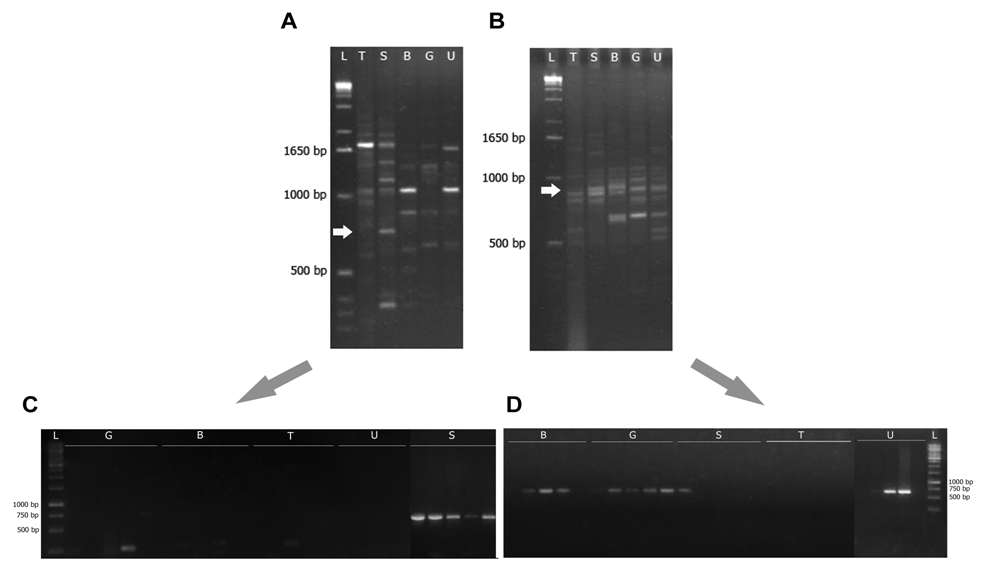
- 59 RAPD primers were tested on two bulks with ten individuals per bulk of E. saligna and E. tereticornis to select a set of RAPD primers that produced reliable and reproducible fingerprints for these two species. Reproducibility of the amplification pattern was checked by repeating each reaction at least twice without alteration in the protocol.Only two RAPD primers (OPAD-01 and OPH-03; Table 2) among the 59 tested produced RAPD patterns that allowed differentiation of the E. saligna and E. tereticornis species. Selection criteria was a sufficient DNA length in order to maximize the availability of convenient sites for designing PCR primers and a well-defined DNA bands to increase the chance of cloning the targeted molecular marker.
|
4. Conclusions
- A set of molecular markers (CAS and CHT) specific to E. saligna and E. tereticornis species were designed to distinguish these species. This is a quick and efficient method with high specificity and reproducibility that can be used in seed, seedlings and stocked wood in the management of populations in forest breeding programs.
ACKNOWLEDGEMENTS
- This work was supported by FAPESP, and the authors thank the assistance with sample collection by Suzano Pulp and Paper Company.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML