-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(6): 419-434
doi:10.5923/j.ijaf.20140406.02
Morphological Variability in Argan Seedlings (Argania Spinosa (L.) Skeels) and Its Implications for Selecting Superior Planting Material in Arid Environments
Zahidi Abdelaziz1, Bani-Aameur Fouzia2, El Mousadik Abdelhamid2
1Polydisciplinry Faculty Taroudant, University Ibn Zohr, Agadir Morocco, Laboratory of Biotechnologies and Valorization of Naturals Resources Faculty of Sciences, Ibn Zohr University, Agadir, Morocco
2Department of Biology, Faculty of Sciences, University Ibn Zohr, Agadir Morocco, Laboratory of Biotechnologies and Valorization of Naturals Resources, Faculty of Sciences, Ibn Zohr University, Agadir, Morocco
Correspondence to: Zahidi Abdelaziz, Polydisciplinry Faculty Taroudant, University Ibn Zohr, Agadir Morocco, Laboratory of Biotechnologies and Valorization of Naturals Resources Faculty of Sciences, Ibn Zohr University, Agadir, Morocco.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The argan multi-purpose tree, is often described as an endangered species since several physical and anthropogenic factors reduced the density and surface of argan ecosystem. With the aim of helping to select superior planting material for implementation of conservation activities in arid environments, genetic variability for several morphological characters of stem and root was studied. 12 seedlings per family distributed in randomized complete block were grown for 12 months under nursery conditions. Several morphological characters of stem and root were observed at the end of the experiment. Intra-family variability was more important than inter-family variability for all characters. Higher heritabilities were observed (0.54 to 0.59) for length of the smallest root and stem length. We did not observe any differentiation between the three populations for stem and root characters. Seedlings from drier site appear to have taproots longer; root / stem ratioand ratio of fresh masswere also higher. Seedlings of some families invested more in their taproot, a key organ for Argan seedling survival. Argan is characterized by great variability between individuals of the same family 'half-brother' which gives possibility to select high quality planting material for regeneration and that can adapt to drought conditions especially after transplantation.
Keywords: Argania spinosa, Arid environments, Diversity, Root seedling growth, Stem
Cite this paper: Zahidi Abdelaziz, Bani-Aameur Fouzia, El Mousadik Abdelhamid, Morphological Variability in Argan Seedlings (Argania Spinosa (L.) Skeels) and Its Implications for Selecting Superior Planting Material in Arid Environments, International Journal of Agriculture and Forestry, Vol. 4 No. 6, 2014, pp. 419-434. doi: 10.5923/j.ijaf.20140406.02.
Article Outline
1. Introduction
- Indigenous fruit trees have the potential to contribute towards food security, nutrition health and income generation (Jamnadass et al., 2009) and mitigate environmental degradation in developing countries (Simbo et al., 2012; Cuni-Shanchez et al., 2011). The Argan tree (Argania spinosa) is one of these indigenous fruit, fodder and forest tree endemic to south west of Morocco and arid Mediterranean type area where rainfall occurs in the winter, highly adapted to aridity (Emberger, 1939; Prendergast and Walker, 1992). As is the case of many species, the domestication of this fruit and forest tree can play a prominent role both for the environment preservation, mostly because of encroaching desert threat (Nerd et al., 1994; Simbo et al., 2012; Zahidi, 1997; Zahidi et al., 2013b) and for the overall economy of the region since it is an important resource for wood, oil and fodder (Nouaim et al., 1991). This multi-purpose tree is therefore the foundation for a unique agro-forestry system (De Ponteves et al., 1990). The potential for growth and production capacity of branches and leaves in plants especially at seedling stage represents a necessary step in understanding biology of the species (Ehrenberg, 1989; Laâmouri and Sghaier, 1998). In addition, stem and root growth is specific to each species, reflecting the adaptation of plants to changes in bioclimatic environment (Becker et al., 1983; Gorenflot, 1986; Larsen et al., 1986). In forest species, production of plant able to grow after planting, is crucial for large-scale reforestation and for developing a conservation program of an appropriate level of genetic diversity (Behm et al., 1997; Zahidi and Bani-Aameur, 1998; Zahidi et al., 2013b). Genetic variation is fundamental component, which ensures survival and stability of the forest ecosystems. This variability determines the potential of population to adapt to changing in environmental conditions. Thus genetic characterization of natural forest resources is necessary for better understanding of genetic resources for implementation of conservation activities (Zhang et al., 2004; Zhang et al., 2005; Turna et al., 2006; Xiao et al., 2008). In fact, in three natural populations of argan in south west Morocco a great variation in growth and fruits productivity has already been reported for trees in the fields (Zahidi, 1997; Zahidi et al., 2014). We distinguish five morphological types of tree habit. In addition growth, branching and leaves production were higher in humid season than in dry season. In three natural populations of argan in south west Morocco a great variation in argan tree growth has already been reported (Zahidi, 1997; Zahidi et al., 2013a). We distinguish five morphological types of tree habit. In addition, growth, branching and leaves production were higher in humid season than in dry season. The relative contribution of genotype (tree / locality) and tree x environment interaction in the total variance was more pronounced (22.2% to 54.3%) for most traits. Individuals from the driest provenance were most affected by inter-annual climate changes; this site through its arid climate can create an environment for selection of resistant genotypes to drought. Argan in the fied is characterized as a slow-growing tree since shoot length even in humid season reached about 30 cm (Zahidi et al., 2013c). But V.A. mycorrhization increased total shoot length, stem growth and biomass of the plants, when cultivated in controlled conditions (Nouaim and Chaussod, 1994). At the present time, studies on variability of growth in argan seedlings are absent. This study aims to investigate the diversity in Argan seedling growth and estimating genetic parameters at nursery stage in order to select high quality planting material for implementation of conservation activities and artificial regeneration.
2. Materials and Methods
2.1. Plant Material
- Kernels are from mature fruits harvested in summer from three geographical origins in south west Morocco, Ait Melloul (AM), Argana (AR) and Ait Baha (AB) (Zahidi, 1997; Bani-Aameur, 2004). Almond kernels from 29 families in each site, with a total of 87 families, are germinated and transplanted according to experimental protocol described previously (Zahidi and Bani-Aameur, 1998a). At the beginning of the experiment, each of 87 families was represented by four transplanted seedlings per block, 20 seedlings within 5 blocks. Due to losses of seedlings (Zahidi and Bani-Aameur, 1998b), we were content to ten families per provenance and only three blocks (12 seedlings per family per geographic origin).
2.2. Methodology
- Seedlings from the three provenances were grown for 12 months. At the end of experiment, several characteristics were recorded from each seedling (Figure 1):
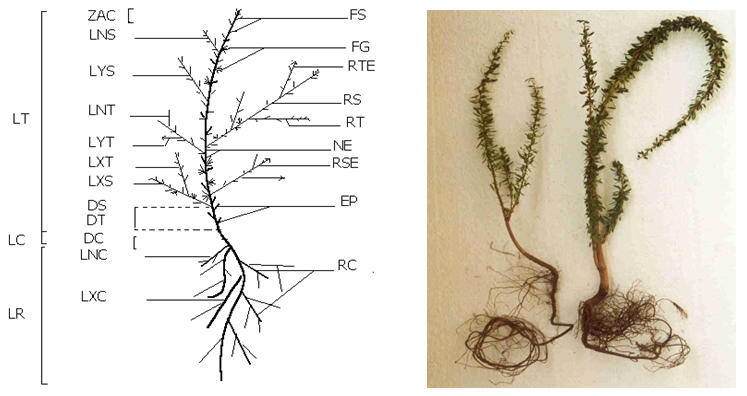 | Figure 1. Morphological characters of the stem and root observed in Argan seedlings grown in nursery conditions for 12 months |
2.3. Statistical Analyzes
- Analysis of variances (ANOVA) with three factors in hierarchical model was adopted. Factor family (seedlings from the same tree) is hierarchical to provenance factor, given that families are not repeated between localities, and within each geographic origin. Factors block and geographic origin are crossed. Comparisons were made with recourse to LSD (method of least significant difference) using a significance level of 95% (Sokal and Rolf, 1995). Variance components were estimated from the appropriate linear functions of mean squares (Lentner and Bishop, 1993) (Table 1).
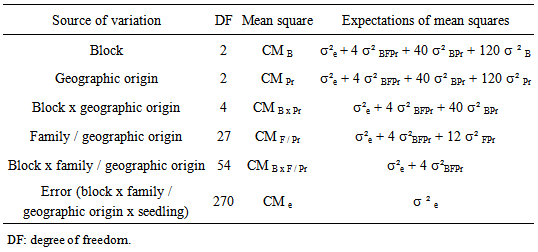 | Table 1. Expectations of mean squares and estimated variance components of the stem and root growth traits of Argan seedlings |
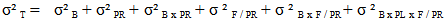
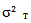 : total variance;
: total variance; 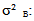 variance due to block;
variance due to block; 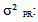 variance related to geographical origin;
variance related to geographical origin; 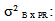 variance due to block x geographic origin interaction;
variance due to block x geographic origin interaction; 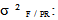 variance due to family / geographic origin (inter-family variance);
variance due to family / geographic origin (inter-family variance); 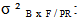 variance due to block x family / geographic origin interaction;
variance due to block x family / geographic origin interaction; 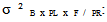 intra-family variance: genetic variance due to differences between seedlings of the same family (error).In heritability estimate, the fact that seedlings of each family are from kernels collected separately in each of the three sites and families are not repeated between provenances and at the same geographical origin. Variance component associated to family factor is confounded with genetic variation between provenances. Narrow sense heritability is calculated using the following model (Gale et al., 1988; Corneluis et al., 1996; Wheeler et al., 1995; Ward, 2001):
intra-family variance: genetic variance due to differences between seedlings of the same family (error).In heritability estimate, the fact that seedlings of each family are from kernels collected separately in each of the three sites and families are not repeated between provenances and at the same geographical origin. Variance component associated to family factor is confounded with genetic variation between provenances. Narrow sense heritability is calculated using the following model (Gale et al., 1988; Corneluis et al., 1996; Wheeler et al., 1995; Ward, 2001):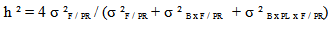
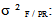 expresses inter-family variance (variance due to family / geographic origin),
expresses inter-family variance (variance due to family / geographic origin), 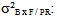 denotes variance related to block x family / geographic origin interaction,
denotes variance related to block x family / geographic origin interaction, 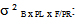 means intra-family variance (variance due to differences among seedlings within the same family). Factorial discriminate Analysis (AFD) was performed on averages of each family in order to examine the simultaneous contribution of all parameters studied in discriminating families and provenances (Bernstein and al., 1988). Dendogram was built using clustering method UPGMA "pair-group method unweighetd arithmetic average". Statistical treatments were performed using Statitcf, Statistix software and Ntsys version 1.40 (Rolf, 1988).
means intra-family variance (variance due to differences among seedlings within the same family). Factorial discriminate Analysis (AFD) was performed on averages of each family in order to examine the simultaneous contribution of all parameters studied in discriminating families and provenances (Bernstein and al., 1988). Dendogram was built using clustering method UPGMA "pair-group method unweighetd arithmetic average". Statistical treatments were performed using Statitcf, Statistix software and Ntsys version 1.40 (Rolf, 1988).3. Results
3.1. Variability Characterization
3.1.1. Block Effect
- + Stem, root growth and branching Block factor was significant for most characters of the root and stem growth and branching (Table 2). + Biomass production Block effect was highly significant for stem (PST) and root (PSR) dry weight, ratio of fresh (RPF) and dry weight (RPS). It was not significant for stem (PFT) and root (PFR) fresh weight.
3.1.2. Geographic Origin Effect
- + Stem growth and branching Geographic origin was not significant for all traits (Table 2). Block x geographic origin interaction was not significant for all characters except length of the greatest secondary branch (LXS). + Root growth and branching Block x geographic origin interaction was significant for LR and LNC, but not significant for the other traits (Table 2). Geographic origin factor was highly significant for LR and RL, but not significant for the remaining characters. Ait Baha population showed longest roots and higher ratio of lengths than Argana and Ait Melloul populations. Thus, main root lengths were between 10.7 to 138.4 cm in Ait Baha, 20.5 to 114.4 cm in Argana and between 20.2 and 132.4 cm in Ait Melloul. Ratio of length (RL) also varied in the same way in the three geographical origins. There were between (6.13 to 0.7) in Ait Baha, (3.25 to 0.48) in Argana and (6.35 to 0.45) in Ait Melloul (Table 3). + Biomass production Block x geographic origin interaction was significant only for root fresh weight. Geographic origin factor was not significant for all traits except fresh weight ratio (RPF) (Table 2). Seedlings in Ait Baha had the highest fresh weight ratio (0.51 and 1.91) whereas they had the lowest fresh weight ratio in Ait Melloul (1.23 to 0.51) and Argana (1.18 to 0.45) (Table 3).
3.1.3. Family / Geographic Origin
- + Stem growth and branchingBlock x family / geographic origin interaction was significant for all traits except number of tertiary shoots. Factor family / geographic origin was significant for most characters of the stem (Table 2). Highest variability was observed among families according to variation intervals of maximum, minimum, average and coefficient of variation (Table 3). Thus, there were two classes of genotypes First class consisting genotypes such as, family (1) of Ait Melloul, families (3 and 7) from Argana, and family (7) of Ait Baha whose stems are longer, with a high number of simple, grouped leaves (Table 4a). In this first class, for the branched seedlings, most shoots are second order. Tertiary branching is very low. In addition, secondary shoots length was important as was the case of families (1) from Ait Melloul, (3) of Argana and family (5) of Ait Baha. Lengths of the greatest shoots were respectively 32.5, 44.3 and 42.1 centimeters. These lengths constitute 48%, 69.4% and 58.1% (LXS x 100 / LTF) of the main stem. Second class includes genotypes from mother-trees such as family (4) from Ait Melloul, family (8) from Argana and family (8) from Ait Baha whose stems are relatively short, with a smaller number of simple and grouped leaves. Branching is very low, when it exists, it is mainly second order. The lateral shoots length was also low. + Root growth and branching Block x family / geographic origin interaction was highly significant for all traits of the below-ground part. Family / geographic origin was significant for LR, RL, RCS and LRCS (Table 2). It was not significant for the remaining characters. Large variability was also found between families (Table 3). Root length was between 10.7 and 138.4 cm, root / shoot length ratio was between 0.45 and 6.35, number of secondary roots varied from 4 to 56. For these characters, two categories of genotypes are also distinguished (Table 4b). The first category consists mother-tree genotypes such as families (6) from Ait Melloul, (6) from Argana, and (2) of Ait Baha whose main root was longer, with considerable number of secondary roots, and whose ratio lengths are higher. If the family (1) of Ait Melloul, and family (7) Argana had relatively shorter roots (average about 45.9 and 52.14 cm), they instead exhibit high number of secondary roots about (18.6 and 24.3 units). Second group of genotypes contains family (3) from Ait Melloul, family (8) from Argana, and family (8) of Ait Baha whose main roots remained shorter, with small number of secondary roots and whose ratio lengths are among the lowest except for family (8) of Ait Baha.+ Biomass production Block x family / geographic origin interaction was significant for fresh and dry weight of the stem and the root and their ratio. Family / geographic origin was significant for PFT, PFR, PST and PSR (Table 2). It was not significant for RPF and RPS. For these traits, there was high level of variation between families (Table 3). We distinguish first class of genotypes consisting families such as (1) from Ait Melloul, family (7) from Argana, and family (7) of Ait Baha, whose fresh and dry weights of the stem and the root are most important. Second class of genotypes consisting families such as (3 and 6) from Ait Melloul, (8) from Argana, and (8) of Ait Baha, whose fresh and dry weights of the stem and the root are lower (Table 4b).
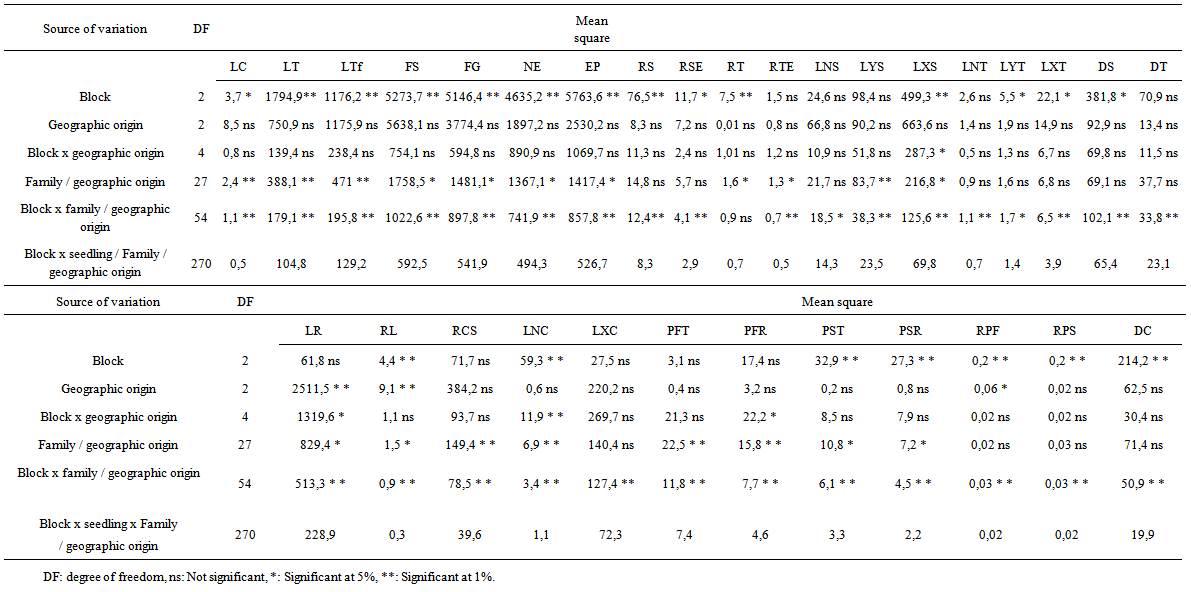 | Table 2. Analysis of variance of the stem and root growth and branching traits of Argan seedlings grown for 12 months |
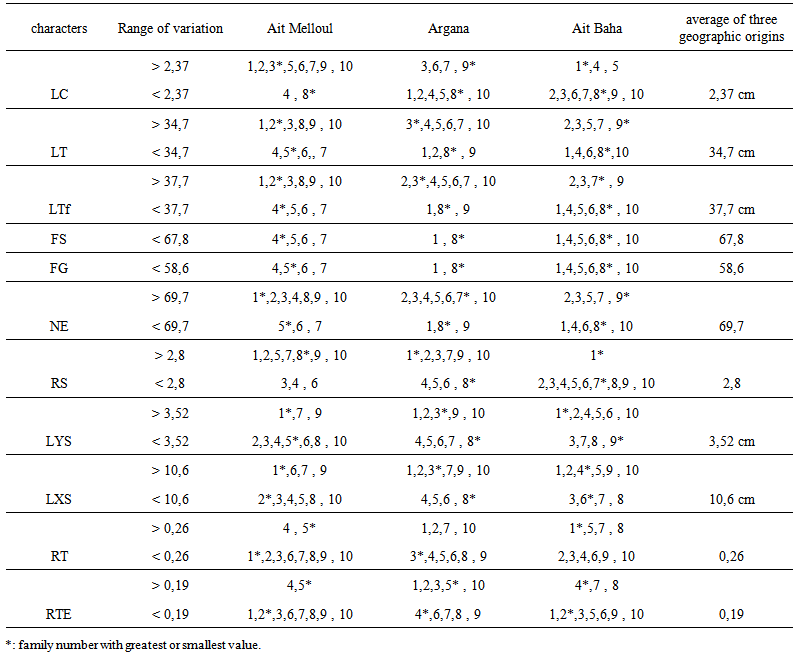 | Table 4a. Range of variation compared to average of three geographic origins, family number for the stem traits in Argan seedlings |
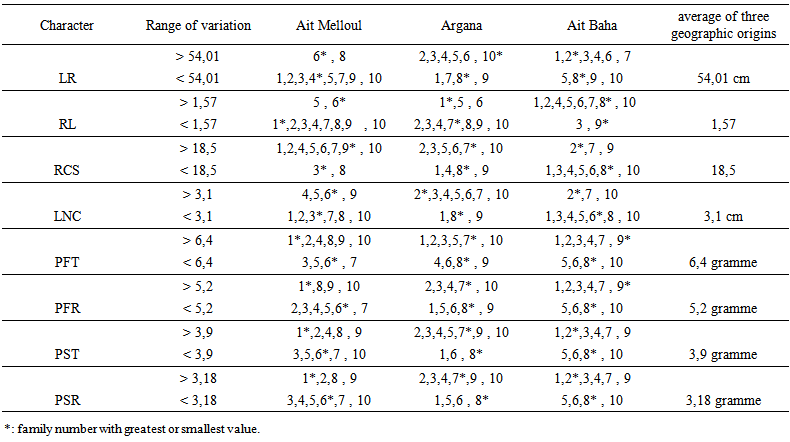 | Table 4b. Range of variation compared to average of three geographic origins, family number for the root characters in Argan seedlings |
3.2. Variance Components
- Relative contribution related to block, geographic origin and block x geographic origin interaction in the total variance was low (0% and 16.2%) for all stem and root traits (Figure 2). The percentage of variance related to block x family / geographic origin interaction in total variance was low and between 5.1% and 6.6% for the tertiary shoot length and number of tertiary shoot. It was relatively high (8.9% and 25.5%) for the other characters. Great intra-family variability was observed for all traits, since the contribution of variance related to block x seedling x family / geographic origin interaction (intra-family variance related to differences between seedlings of the same family) in the total variance was higher (45.1% and 93.5%). The percentage of variance associated with family / geographic origin (mother-tree genotype which means the inter-family variance) in the total variance was variable and ranged between 0% and 13.1% for all characters. Heritabilities varied in large proportions. The lowest values (0.0 to 0.08) were recorded for RS, LNS, LNT, LYT, LXT, DS, DT, LXC, fresh and dry weight ratios (Table 5). For the other characters, heritabilities in narrow sense were between 0.16 for number of secondary shoot and 0.59 for length of the smallest secondary root.
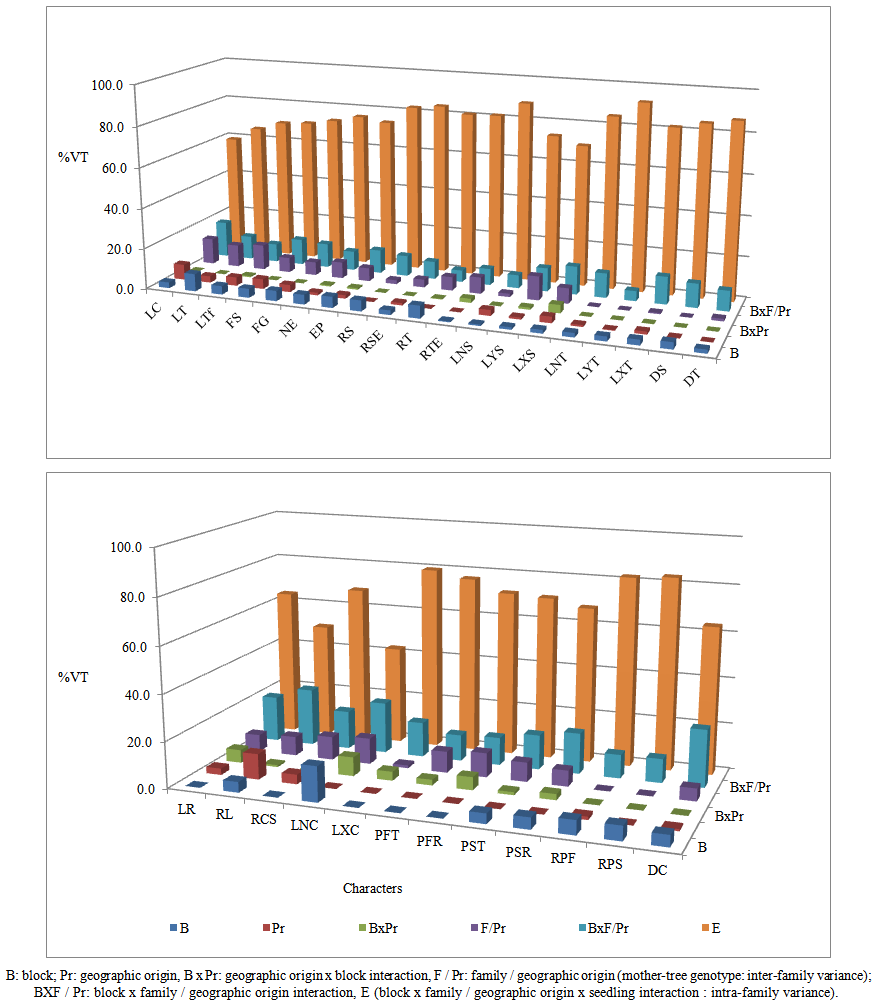 | Figure 2. Percentages of variance components in the total variance (VT) for the stem and root characters in Argan seedlings grown in nursery conditions |
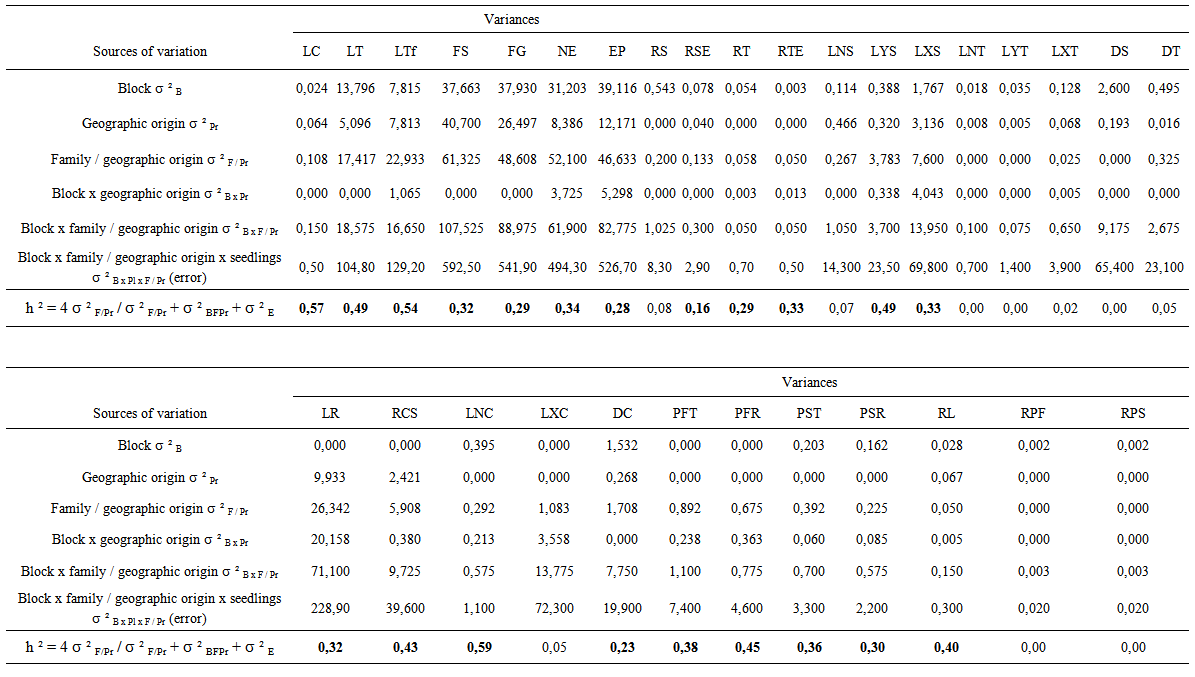 | Table 5. Variance components and heritabilities in norrow sense of the stem and root characters of argan seedlings grown in nursery conditions |
3.3. Diversity Analysis
- Correlation for the stem and the root growth and branching was very marked (Table 6). Stem growth traits such as LT, FS, FG, NE and EP are highly correlated (0.91 and 0.99). For the remaining characters of the stem, correlation was ranged from 0.51 to 0.85. Root characters are not correlated. Fresh and dry weights of the stem and the root are highly correlated (0.84 to 0.96).
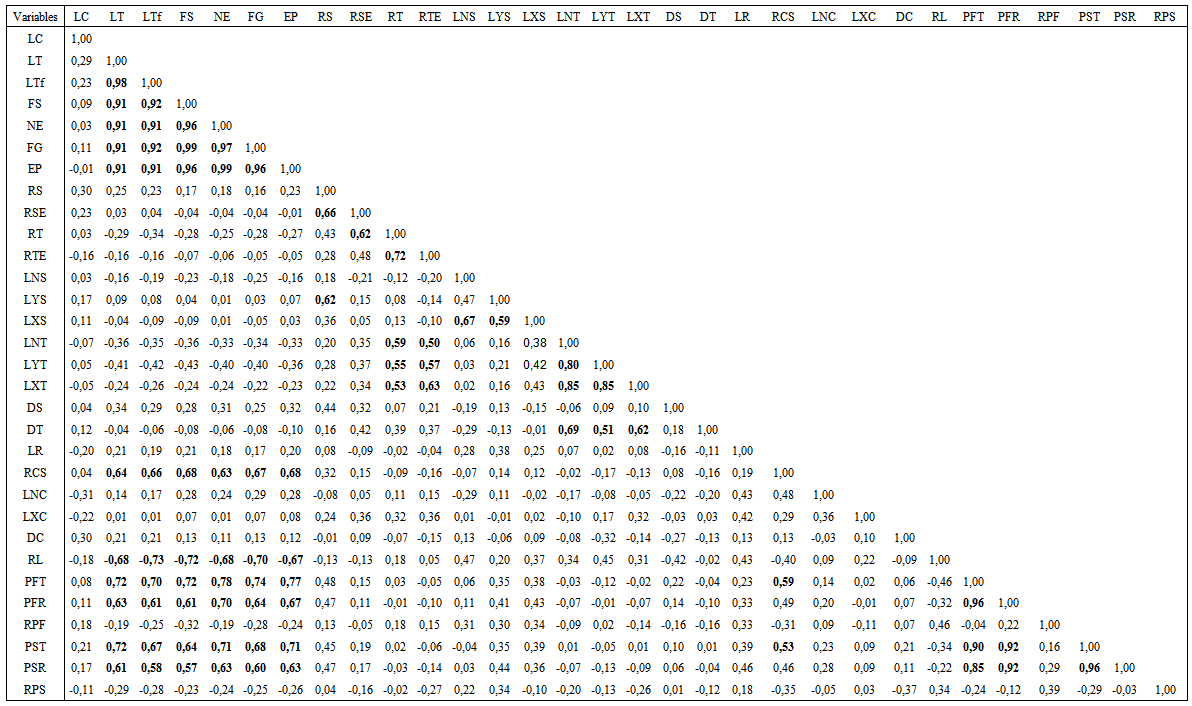 | Table 6. Correlation coefficients among stem and root characters of Argan seedlings grown in nursery for 12 months |
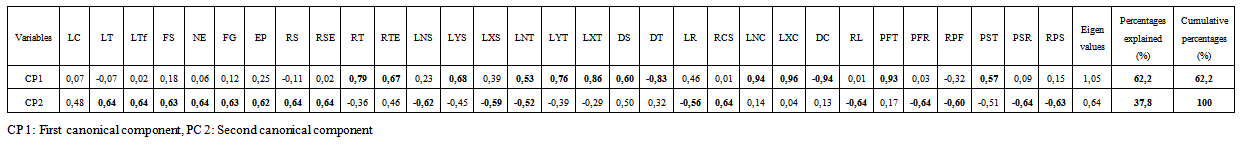 | Table 7. Canonical correlation between the components and characteristics of the stem and root in Argan seedlings grown in nursery for 12 months |
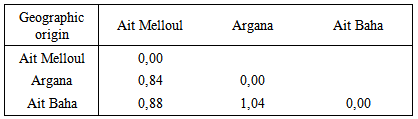 | Table 8. Mahalanobis distance between the three geographic origins for the stem and root growth characters in argan seedlings |
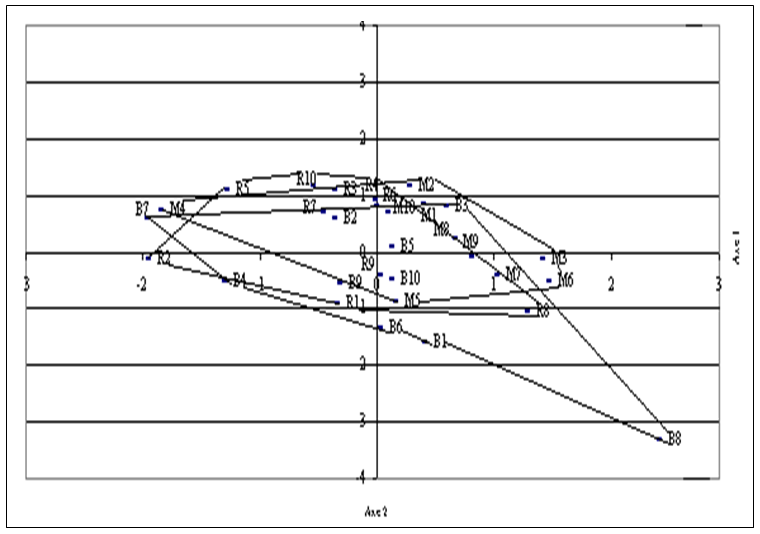 | Figure 3. Representation of families from Ait Melloul (M), Argana (R) and Ait Baha (B) in the plane defined by the first two canonical components |
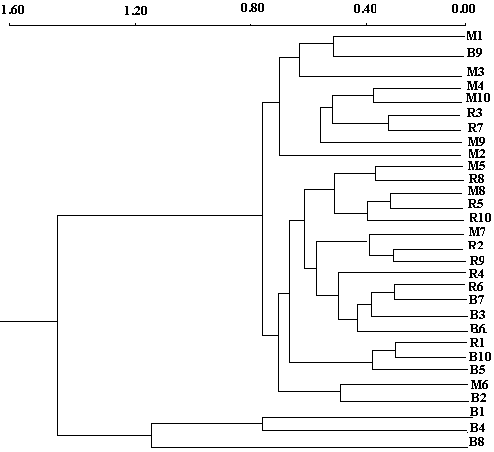 | Figure 4. Cluster analysis classification of families based on growth and branching characters of the stem and root in Argan seedlings at nursery stage |
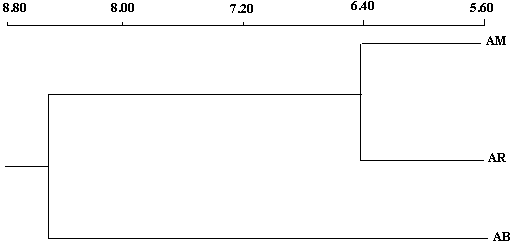 | Figure 5. Cluster analysis classification of geographical origins based on growth and branching characters of the stem and root in Argan seedlings at nursery stage |
4. Discussion
- The analysis of variance revealed a strong morphological variation in argan seedlings for the stem, root characters and biomass production. A wide range of variation was observed between geographical origins in root length, root / stem ratio and fresh weight ratio characteristics. In general, seedlings from drier site, seems to have characteristics often related to drought tolerance than those from wetter provenances. Thus, from drier site Ait Baha 30% and over 60% of families have respectively main roots 2.5 times and 1.5 times longer than the main stems. Seedlings from Ait Melloul, only 10% of families have main roots 2.5 times longer and 30% of them with roots 1.5 times longer than the main stems but with high number of lateral roots. While seedlings from wetter site Argana, 0% and 60% of families have roots respectively 2.5 times and 1.5 times longer than the stem. In addition, seedlings have relatively more water stored in their roots, since its content represents more than 60% of the total root weight in 7/10 families of Ait Baha and Ait Melloul and 6/10 families from Argana. This root system growth will be useful when transplanted to the field, plants achieved rapid deep rooting and root avoided excessive surface ramification. This might help seedlings from drier environments to survive periods of drought stress, which are likely to be more common in drier sites where rainfall is lacking more than 6 months (Ferradous et al., 1996; Zahidi, 1997). Similar findings were observed in other plants (Kundu and Tigerstedt, 1997; Cuni-Sanchez et al., 2011). In these species, seedlings from drier provenances have longer roots and root length / stem length higher. Whereas, seedlings from wetter sites showed shorter roots and smaller ratio lengths, but with a high number of lateral roots. In Eucalyptus microtheca (Li, 1998), seedlings from locality with low rainfall have ratio length (root length / stem length) (0.49) higher than seedlings from watered locality (0.41). Root dry weights varied in opposite direction between the two geographical origins. Natural populations of Argan, was the only representative species of the tropical family Sapotaceae in south west Morocco. Hamrick et al., (1992), reported that woody perennial species maintain generally most of their variation within populations, which holds true for tropical trees in particular. This variation was associated with the life history and ecological characteristics of the woody species. Our result is congruent with such as description, sine the contribution of inter-family and intra-family variance in the total variance was higher than variance related to geographical origin. These two components explain between 58.8% and 93.5% of total variability. Intra-family is much higher than variability between families. It has been reported that percentage of intra-family variance in Jungus hindsii was between 59.5% and 83.1% for the four traits studied (Gale et al., 1988), for height, diameter, fresh weight of stem and root in Tsuga mertensiana (Benowicz and Kassaby, 1999), and in Eucalyptus grandis and E. globulus in which, there is wide difference between families within geographic origins and between families for height and fresh weight of the stem (Marcar et al., 2001). Similar differences were also observed among and within progenies for different growth parameters at the age of 8 years in fifty-four progenies of Melia azedarach selected from 11 geographical locations in India (Meena et al., 2014).In areas where environmental constraints were hard, the creation of adapted varieties and then superior planting material goes through evaluation of genetic diversity level of local landraces. In this study, seedlings from different maternal trees such as family (1) from Ait Melloul, (3 and 7) from Argana, and (7) from Ait Baha have developed the above-ground manifested by long stems, with high number of simple and grouped leaves and able to branch. In the same time, they have developed a below-ground part, with a relatively long main root, a high number of secondary roots sometimes accompanied by strong lateral root development. This case is not always present because growth of the stem and formation of simple and grouped leaves was not always accompanied by root growth, but correlated to ratio of lengths, biomass production (root fresh and dry weight). As reported by Rodríguez et al., (2012) for Mediterranean oaks that mother identity presented stronger effect on seedling performance. In situ, mother-trees genotypes of families (1, 7 and 7) are characterized by longer main branches, longest shoots and important branching since secondary, tertiary and even quaternary branches are present (Zahidi, 1997). These families can be used as germoplasm in breeding program of growth and branching character. Further information on the nature and the degree of genetic diversity present in argan could help to identify elite trees for genetic improvement through hybridization. High heritabilities in narrow sense (0.26 and 0.59) for most characters of the stem and the root can be considered as good genetic markers for selection and identify suitable plants adapted to environmental conditions in arid areas. These values are relatively lower than those observed in Eucalyptus grandis and E. globulus (0.35 and 0.92) for height and fresh weight of the stem (Marcar et al., 2001) and in white spruce seedlings aged 18 weeks for growth in stem length (0.92) (Rweyongeza et al., 2003), and of Pangamia pinnata (Divakara et al., 2010) for seeds traits, heritability is ranged from 0.82 (for seed length) to 0.98 (for 100pod weight); or in seedlings in two provenance–progeny tests of sweet chestnut (Castanea sativa Miller) (heritability is ranged from 0.12 to 0.43) (Miguez-Soto and Fernandez-Lopez, 2014) .Distribution of diversity is not done in relation with belonging of the families to their geographical origin. Mahalanobis distances were smaller between Argana and Ait Melloul, between Ait Baha and Ait Melloul, compared with distances observed for fruiting branch characters (Zahidi, 1997; Zahidi et al., 2013), for fruit and stone characters in the field (Bani-Aameur and Ferradous, 2001). Main groups obtained by UPGMA, include individuals from Ait Melloul, Argana and families of Ait Baha. Thus, differentiation for growth and branching traits in argan seedlings is not established especially among populations geographically close Argana, Ait Baha and Admine as reported by El Mousadik and Petit, (1996a) on the basis of nine isozyme loci in ten populations of Argan area. This distribution pattern was observed also in Taxus brevifolia on basis of morphological characters (Wheeler et al., 1995), and in J. californica and J. hindsii on basis of biometric traits (Gale et al., 1988) and in fourteen accessions of pearl millet (Pennisetum glaucum (L.) R. Br) from Tunisia and West Africa (Skates et al., 2003). In Pinus silvestris, analysis showed that significant differences were observed within populations for morphological characters of seeds and for seedlings one year old (Sevik et al., 2010). In our Case, the grouping obtained based on morphological characteristics were influenced by genome in contrast to results reported by Dinis et al., (2011) in Chestnut tree (Judia variety). Morphological characters studied were not influenced by genome but essentially by the different climatic conditions and potentially differences in the expression of genes associated with the adaptation strategies. At the present time, several physical and anthropogenic factors reduce the density and surface of argan ecosystems, so it decreases the biodiversity in natural area. Due to the continuous intensify of genetic erosion, it is necessary to save this species. Our study identify mother-tree genotypes with superior traits, so a common garden with superior trees in specific traits from several geographical origins could be established to determine if they will be reproduce the trait of interest in different environment as it proposed for the African baobab (Simbo et al., 2012).
5. Conclusions
- Results from this study indicate that there is a great morphological variability in Argan seedlings at nursery stage for growth and branching characters of the stem and root. In order to protect the natural population and at the same time meet the demands of the local communities in fruit, oil, forage and wood, the argan tree should be domesticated. Our analysis provides information on the nature and the degree of genetic diversity present in argan which could help to identify elite trees for genetic improvement through hybridization. In fact, seedlings from drier provenances allocated more resources to their main roots expressed by strong growth in length, but a few number of lateral roots. This ability to develop long roots even if they are grown under nursery conditions seems to be drought adaptation criteria. This drier provenance is an environment selective of resistant genotypes to drought. Further research is thus needed to address the relationship between seedling establishment and field magangement pratices in order to select drought tolerant planting material for conservation and regeneration in future breeding programs.
ACKNOWLEDGEMENTS
- We gratefully acknowledge anonymous reviewers and office journal which provided helpful comments that greatly improved the manuscript. We thank the Morocco-Germany Co-operative Project ‘Conservation Project and Development the argan forest’(PCDA-GTZ) and the project Pars-Agro 128 of the Morocan Ministery of Scientific Research and the National Agency for the Development of oases areas and of the argan tree (ANDZOA) for financial support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML