-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(5): 373-379
doi:10.5923/j.ijaf.20140405.05
Fungi Associated with the Postharvest Fungal Deterioration of Shea Nuts and Kernels
Esiegbuya D. O.1, Osagie J. I.1, Okungbowa F. I.2, Ekhorutomwen E. O.1
1Plant Pathology Division, Nigerian Institute for Oil Palm Research (NIFOR), Benin City
2Department of Plant Biology and Biotechnology, University of Benin
Correspondence to: Esiegbuya D. O., Plant Pathology Division, Nigerian Institute for Oil Palm Research (NIFOR), Benin City.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The preliminary investigation into the postharvest fungal deterioration of Shea nuts and kernels with the aim to investigate and assess the impact of the fungi on the postharvest deterioration of Shea nuts and stored Shea kernels revealed four types of postharvest deterioration which are nut cracks, kernels discoloration, nuts discoloration and kernel deterioration. The major causes of the postharvest deterioration of Shea nuts are mainly as a result of cracks in the nut, insect’s larvae and microorganisms. The microorganisms isolated from the postharvest fungal deterioration of Shea kernels using the serial plate dilution techniques, revealed the presence of four genera of microorganisms such as Aspergillus, Mucor Phoma and Fusarium spp while two genera of microorganisms namely Aspergillus and Xylaria were isolated from the Shea kernels and nuts respectively. The prevalence of these fungi on the deteriorated Shea kernels was as a result of favourable pH of between 5.8 and 6.0 and moisture content of between 4.76% and 10%. The possible source of these microbial contaminants on the Shea nuts and kernels was through cracks in the Shea nuts, insect’s larvae, contaminants from the environment and storage conditions. The results of the pathogenicity test with these isolates showed that Aspergillus niger, A. flavus and A. persii were more pathogenic on the Shea kernels than the other isolates. These pathogens caused loss of viability of Shea nuts, tainting, colour deterioration and rot of the kernels which could result in poor quality of the processed Shea butter. Due to the economic importance of the Shea nut and kernels which include it uses for Shea butter and Shea oil production, it is important to development an appropriate cost effective techniques for storing the Shea kernels against postharvest fungal deterioration so as to harness its full economic benefits.
Keywords: Shea kernels, Postharvest disease, Fungal deterioration, Shea butter
Cite this paper: Esiegbuya D. O., Osagie J. I., Okungbowa F. I., Ekhorutomwen E. O., Fungi Associated with the Postharvest Fungal Deterioration of Shea Nuts and Kernels, International Journal of Agriculture and Forestry, Vol. 4 No. 5, 2014, pp. 373-379. doi: 10.5923/j.ijaf.20140405.05.
Article Outline
1. Introduction
- The Shea tree recently has gained importance as an economic crop because of the soaring demand for its butter, both domestically and internationally. The Shea tree also known as Vitellaria paradoxa, widely known under its synonym Butryospermum paradoxum belongs to the Sapotaceae family. It is considered a monotypic genus with two subspecies stretching nearly 5,000 km across the African savanna (Marnaz et al., 2004; Fobil, 2010).Shea fruits serve as an important source of food for many organisms and other animals including birds, bats, elephants, sheep and pigs (Marnaz et al., 2004 and Fobil, 2010). The fruits also contribute to food security in forest areas of Nigeria, mainly for the rural poor, especially since their ripening happen together with the lean season of food production (Fobil, 2010). It plays a crucial role in its highly nutritional capacity, food security and availability during the period of low food availability. The production of Shea nut and processing of the butter serves as one of the main sources of employment for the rural women and children who are engaged in gathering it. Shea butter is important edible oil for the people of Southwestern Nigeria and most of Western Africa, being the most essential source of fatty acids and glycerol in their diet (Saul et al., 2003; Moore, 2008). It is also useful in the pharmaceutical and cosmetic industries as an important raw material and a precursor for the manufacture of soaps, candles, and cosmetics (Fobil, 2010).Shea nuts are also more and more being exported for the utilization in the cosmetics industry as a constituent in lotions, makeup, baby ointments, hair care products and soaps (Akosah-Sarping, 2003; Moore, 2008). Regardless of being increasingly substituted by commercially produced lotions in many communities, Shea butter is traditionally used as a skin and hair moisturizer and for protection against the sun (Ezema and Ogujiofor, 1992). The healing properties and effects of Shea butter are thought to be somewhat attributable to the existence of allantoin, a substance known to trigger the growth of healthy tissue in ulcerous wounds (Wallace-Bruce, 1995). It is also traditionally smeared on pregnant women during childbirth, on new born babies and adolescents because of its relieving effects (Moore, 2008). Though the nuts being an essential export commodities, its fruit pulp nevertheless is widely consumed. It also plays a major role in the local economy and diet as well as occupies an important period of time in the annual local dietary cycle (Maranz et al., 2004). The edible part of the Shea fruit is extremely nutritious and has important nutrients for the human body. The fruit provides important source of food for communities and rural poor particularly at the period of food shortages, hunger and other catastrophes. In addition to providing health benefits, it also provides some income (FAO, 2007).Microorganisms associated with Shea pulpbio-deterioration includes; Lasiodiplodia sp., Botryospahaeria sp., Pseudofusicocum sp., Colletotrichum gloesporioides, Pestalotiopsis sp, Phoma sp and Phomopsis (Sakalidis et al., 2011). Most of these pathogens have also been reported from other parts of the world to cause Shea butter fruit rot which could result in its fruit losses. This study was therefore carried out to investigate and document the fungi responsible for the postharvest fungal deterioration of Shea nuts and stored Shea kernels.
2. Material and Methods
2.1. Source of Shea Nut and Kernels Material
- The Shea nuts and kernel used in this study were obtained from Shea farmers and processors in Kwara, Niger and Oyo states of Nigeria.
2.2. Detection of Postharvest Fungal Deterioration
- Shea nuts and kernels collected were examined for postharvest fungal deterioration by visible inspection for the presence of (1) Blackish colouration of the nuts and kernels (11) Cracks and holes in the nuts and kernels (111) Mould growth on the surface of the nuts and kernels.
2.3. Isolation of Fungi from the Shea Nuts and Kernels
- Six McCartney bottles label10-1 to 10-6 and the stock bottle labelled S1 were prepared in triplicate and then sterilized in an autoclave at 121℃ for 15 minutes. The stock bottle labeled S1 was prepared using the macerating the Shea nuts and kernels in a sterilize Petri dish. 1g each of the macerated Shea nut and kernel was weighed into each of the labeled stock bottle before it was then make up to 10mls by adding sterilize distilled water. The stock bottle was swerved and allowed to soak for 30minutes so that the microorganisms can dislodge into the waters. With a sterile pipette, 1ml each was transferred from the stock bottle into the bottles labelled 10-1 to 10-6 containing 9mls of sterilized distilled water for serial dilution preparation. From the labeled McCartney bottles 10-5 and 10-6, 1ml of the samples were pipetted into a Petri dish, after which, prepared potato dextrose agar were poured into each of the Petri dishes. Petri dishes were incubated on a laboratory bench at laboratory temperature of 28 ± 2℃ for 3-7 days. After the period of incubation, the different colonies of fungi that grew on the plates were each aseptically subcultured using a flamed inoculating loop, into a sterile plate containing fresh PDA. The frequencies of the various colonies were recorded. Colony forming unit for each isolates were also recorded by counting the number of propagules forming unit for each isolates (Sekar, et al., 2008).
2.4. Molecular Identification of Isolates
- Molecular identification of the fungal isolates was carried by the International Mycological Institute, Surrey, UK. Molecular identification was carried out on the samples using nucleic acid as a template. A proprietary formulation [microLYSIS®-PLUS (MLP), Microzone, UK)] was subjected to the rapid heating and cooling of a thermal cycler, to lyse cells and release deoxyribonucleic acid (DNA). Following DNA extraction, Polymerase Chain Reaction (PCR) was employed to amplify copies of the ITS fragment of rDNA in vitro for filamentous fungi. The quality of the PCR product was assessed by undertaking gel electrophoresis. PCR purification step was carried out to remove unutilised dNTPs, primers, polymerase and other PCR mixture compounds and obtain a highly purified DNA template for sequencing. This procedure also allowed concentration of low yield amplicons.For all samples, sequencing reactions were undertaken using BigDye® Terminator v3.1 kit from Applied Biosystems (Life Technologies, UK) which utilises fluorescent labelling of the chain terminator ddNTPs, to permit sequencing. Removal of excess unincorporated dye terminators was carried out to ensure a problem-free electrophoresis of fluorescently labelled sequencing reaction products on the capillary array AB 3130 Genetic Analyzer (DS1) DyeEx™ 2.0 (Qiagen, UK) modules containing prehydrated gel-filtration resin were optimized for clean-up of sequencing reactions containing BigDye® terminators. Dye removal was followed by suspension of the purified products in highly deionised formamide Hi-Di™ (Life Technologies, UK) to prevent rapid sample evaporation and secondary structure formation. The samples were loaded onto the AB 3130 Genetic Analyzer and sequencing undertaken to determine the order of the nucleotide bases, adenine, guanine, cytosine, and thymine in the DNA oligonucleotide. Following sequencing, identification was undertaken by comparing the sequences obtained with those available at the European Molecular Biology Laboratory (EMBL) database via the European Bioinformatics Institute (EBI).
2.5. Determination of the pH and Moisture Content of Shea Kernels
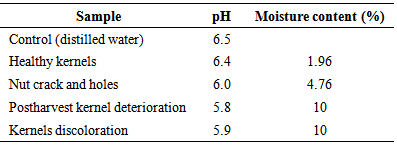
- The pH and the moisture content of the Shea kernel was determined by initially using a local mortar and pestle to grind the kernels into a powdered form. Ten gram of the powdered Shea kernel was weighed using a weighing balance and then transferred into a conical flask. The powdered Shea kernel sample in the conical flask was then mixed with 30mls of sterile distilled water. The mixture was stir using a magnetic stirrer Measurement of pH was carried out on pH meter (model 7020 Electronic Instrument Limited Kent).The moisture content of Shea kernel was determined using the official methods of analysis (AOAC 1990).Two grams of the Shea kernel sample was weighed into a previously weighed crucible. The crucible containing sample was then transferred into the oven set at 100℃ to dry to a constant weight for 24 hours overnight. At the end of the 24 hours, the crucible with sample was removed from the oven and transferred to dessicator, cooled for 10 minutes and weighed.If the weight of empty crucible = Wo Weight of crucible plus sample = W1 Weight of crucible plus oven-dried sample =W3
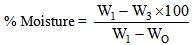
2.6. Preparation of Spore Suspension
- Spore suspension was prepared for the purpose of inoculation. The experimental plant materials, spore suspension of each isolate was prepared according to the method of Krupinsky (1981). Four to seven days old PDA plate cultures of isolate (depending on how fast the fungus grows) were blended in waring blender in 500 ml of sterile distilled water. The spore suspension was filtered through two layers of cheese cloth into a conical flask. A spore suspension of spore load from 1.6-6.5×10-5per ml was used as standard for pathogenicity test inoculations.
2.7. Pathogenicity Test
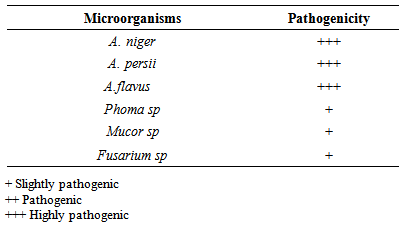
- The most occurring fungi associated with the postharvest fungal deterioration were tested for pathogenicity. Healthy Shea kernels were surface sterilize by soaking the kernels in 0.1% mercuric chloride for 3 minutes and then washed in five changes of sterile distilled water to remove surface contaminants. The surface sterilized Shea kernels were divided into four batches. Each batch was transferred into a moistened transparent sterile container (500 mls) containing the spore suspension of each fungal isolates. After incubating for 5 to 14 days, isolation was carried out on the inoculated Shea kernels. The fourth batch of the Shea kernels was treated as above except that; they were soaked in sterile distilled water before transferring into the moistened beaker. This served as a control. This experiment was repeated twice.
3. Results
3.1. Types of Deterioration caused by Fungi on Shea Nuts and Kernels
3.1.1. Nuts Crack and Holes
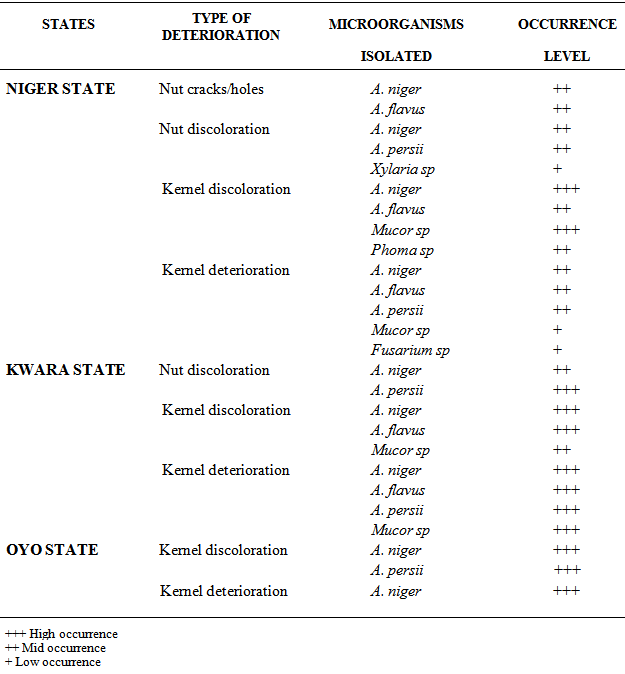
- The crack and holes noticed in the Shea nut (Plate i ) in the study was not attributed to fungal infection. The crack in the Shea nut was attributed to mechanical injury during rainfall and heat from sunlight. Tiny holes on the surface of the nuts were attributed to insects’ infestation which has the ability to burrow hole through hard surfaces. However, no insect was intercepted during the course of this study. Cracks and holes in the nuts paved way for microorganisms such as Aspergillus niger and A. flavus into the kernels. These microorganisms were isolated from the nuts with cracks and holes during the course of this study.
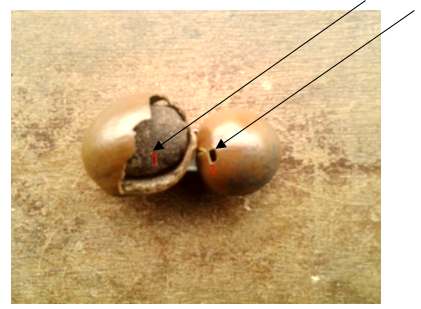 | Plate 1. Exposed shell nuts showing (1) cracked nut (2) hole in the nut |
3.1.2. Kernel Discoloration
- Healthy kernels are dark brown in colour, with no signs of mould growth, cracks, and discolouration (Plate vii). The discolouration of the Shea kernels (Plate B) was as a result of microscopic fungi which are themselves in different in colour, thereby impact different colour on the kernels where they grow thereby changing their appearances. Microorganisms such as A. niger, A. flavus, Mucor and Phoma sp were isolated from the kernel deterioration.
 | Plate 2. Stored shell kernels showing signs of mould growth |
3.1.3. Nut Discoloration
- Healthy Shea nuts are coffee brown in colour (Plate vi). The colour discoloration of the Shea nuts (Plate iii and iv) was a result of microscopic fungi which are themselves in different in colour, thereby impact different colour on the nuts and kernels where they grow thereby changing their appearances. The black and white colour discolouration on the nut (Plate iii and iv) and was as a result of black and white mould contamination caused by the various species of Xyalria, A. persii and A. niger isolated from the nut surface. These pathogens resulted in the damage of the endosperm of the nut, thereby making the nut unsuitable for planting.
 | Plate 3. Black mould coloration of Shea nut |
 | Plate 4. Whitish mould on the Shea nuts and damage testa |
 | Plate 5. Kernels with holes and insects larvae |
 | Plate 6. Healthy Shea nuts with healthy testa |
 | Plate 7. Healthy Shea kernels |
3.1.4. Kernel Deterioration
- Kernels deterioration was as a result of mites, injury in the kernels (during the nut cracking stage in the value chain) created opportunity for insects larvae which to burrows holes into the kernels as shown on Plate v and microorganisms such as A. niger, A. persii, A. flavus, Phoma and Fusarium spp were isolated from the kernel deterioration.
3.2. Results of Microorganisms Isolated from Postharvest Fungal Deterioration of Shea Kernels
- The results of the microorganisms associated with the different types of postharvest deterioration of Shea nuts and kernels collected from Niger, Kwara and Oyo States showed on Table 1 below, revealed the presence of mainly four genera of microorganisms which are Aspergillus, Mucor, Phoma and Fusarium spp. Aspergillus persii, A. flavus and A. niger were mainly the predominant organisms in all the samples analyzed while Mucor, Phoma Fusarium and Xylaria spp were the least predominant. Xylaria sp was isolated only from the Shea nut discoloration showing whitish mycelia growth obtained from Niger State (Table 1 and Plate iv).
|
|
|
4. Discussion
- The Shea nuts are usually sun dried for 1-2 weeks and dehusked to obtain the Shea kernel. Methods of solar drying on polythene sheeting have been developed in some African countries, but they have limited durability (FAO and CFC, 2005). According to USAID (2004), the Shea kernels can be stored for several years without spoilage by maintaining its moisture content between 6% and 7%. This is so because the drying process inactivates enzymes responsible for the build-up of fatty acids in the seed kernel (USAID, 2004). In the study, the moisture content of the Shea kernels with postharvest deterioration and discoloration was 10%, which has presumably contributed to the high incidence of the isolated fungi. The dried Shea nuts obtained were observed have to have cracks and holes. The possible causes of these cracks and holes in the Shea nut and kernels during drying and depulping stage of the Shea processing, might be due to mechanical injury during the depulping of the Shea fruit and insects larvae. The larvae have the ability to bore holes through the nuts and kernels thus exposing the nuts and kernels to microbial contamination during the drying stage. It has been reported by Mlambo, et al., 1992 and Agboola, (1992) that species of insects that are associated with various stored seeds in Nigeria and other parts of Africa cause appreciable damage to stored rubber seeds by boring holes in the kernels, after gaining entrance through the micropyle, in the hard testa.. Shea kernels are stored in sacks, woven baskets and plastic buckets that are kept either in the house, granary or kitchen floors. Sometimes the kernels are hung in houses or kitchens instead of floors. In West Africa Jute bags from cocoa industry are widely applicable (FAO & CFC, 2005). Over the past decades, polythene bags or sacks have come into wide use for storage of Shea kernels. However, this has been reported to stimulate fungal growth because they do not allow air circulation (FAO & CFC, 2005). In this study, samples of the Shea kernels were obtained from sacks, which did not allow for air circulation and as a result, contributed to the high incidence of the postharvest fungal deterioration of the kernels. Some of the kernels obtained from the sack were healthy while some had undergone a form of deterioration. The deterioration observed in kernels obtained from the sack was as a result of the favourable moisture content of the kernels. Healthy Shea nuts and kernels are coffee brown in colour, their discoloration to black/dark colour is as a results of the activities of postharvest fungi. According to JICA, (2007) Shea nuts will turn black if the nut are not dry well or if they are wetted by rain or when direct sunlight is not available. Poorly dried or black nuts fetch lower prices on the market than well-dried kernels.According to Igeleke and Ekpebor, (1986), fungi such as Aspergillus flavus and niger growing on Palm kernels are known to impact various colour to the seeds and also increase the free fatty acids content. Aspergillus niger and A. persii, A. flavus which were isolated in this study, are among the most abundant and widely distributed organisms on earth (Bennet and Klich, 2003). Their main impact on agriculture is in saprophytic degradation of products before and after harvesting and in the production of mycotoxins (Domsh et al., 1980). Aspergillus niger, which was one of the species that showed the highest proportion of pathogenic property on the Shea kernel in this study, has been reported as a major contaminant of peanuts, corns, grains (Cheesborough, 2005) and most popular staple foods in Africa (Thomas et al., 2012). Members of this group as indicated in the CABI identification report from this study are common and widespread. They have been reported to produce mycotoxins including malformin and naphthopyrones and some strains are known to produce ochratoxin. Members of this species aggregate have been implicated in human and animal infections including superficial and local infections (cutaneous infections, otomycosis, tracheobronchitis), infections associated with damaged tissue (aspergilloma, osteomyelitis), pulmonary infections and clinical allergies (allergic bronchopulmonary aspergillosis, rhinitis, Farmers’s lung). However, the majority of infections relate to immunocompromised individuals while the Aspergillus flavus strain has been reported to produce both aflatoxins B and G. (Klich, 2002). Other fungal species, isolated, in this study have also been linked with different types of nut at one time or the other (Thomas et al., 2013). However, the Xylaria sp isolated from the Shea nut in this study was morphologically similar to that reported by Esiegbuya et al., (2013) to cause the postharvest dry rot disease on Raphia hookeri fruits. The organism has the ability to thrive well on dry fruit surface. This is as a result of the xerophilous lifestyle of their ancestors as reported by (Rogers 2000). In terms of pathogenecity, all the isolated fungal mycoflora were found to be pathogenic but to varied degrees. This observation, further stressed that the isolated mycoflora are capable of causing different diseases to Shea nuts and kernels.
5. Conclusions
- Due to the economic importance of Shea kernels, It is therefore, very imperative, to develop appropriate storage conditions for it in order to reduce the incidence and occurrence of postharvest fungal deterioration. Particularly, sources of contamination such as cracks in the kernel, contaminated storage bags and environment should be considered in order to prevent loss of viability and deterioration of the Shea nut.
ACKNOWLEDGEMENTS
- The authors’ are indeed grateful to the commonwealth mycological institute (CABI) for the molecular identification of the pathogens and also to the Management of the Nigerian Institute for Oil Palm Research for providing funds for this research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML