-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(5): 365-372
doi:10.5923/j.ijaf.20140405.04
Putative Vectors of a Phytoplasma Associated with Coconut (Cocos nucifera) in Madang Province, Papua New Guinea
Carmel A. Pilotti1, Charles F. Dewhurst1, Lia W. Liefting2, Lastus Kuniata3, Titus Kakul4
1PNG Oil Palm Research Association, P.O. Box 97, Kimbe, West New Britain Province, Papua New Guinea
2Plant Health and Environment Laboratory, Ministry for Primary Industries, P.O. Box 2095, Auckland 1140, New Zealand
3Ramu Agri-Industries Ltd. (NBPOL), Gusap, P.O. Box 2183, Lae, Morobe Province, Papua New Guinea
4PNG Cocoa Coconut Institute, Madang Province, Papua New Guinea
Correspondence to: Carmel A. Pilotti, PNG Oil Palm Research Association, P.O. Box 97, Kimbe, West New Britain Province, Papua New Guinea.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Surveys to identify potential vectors of a phytoplasma associated with coconuts were undertaken at three sites in Madang Province of Papua New Guinea. Sap-feeding insects from the families Delphacidae, Derbidae, Flatidae, Lophopidae and Pentatomidae were found to contain the Banana wilt associated phytoplasma (BWAP). Further research is underway to confirm the vectors of this phytoplasma.
Keywords: Bogia Coconut Syndrome, Insect Vectors, Phytoplasma, Banana-Wilt Associated Phytoplasma (BWAP)
Cite this paper: Carmel A. Pilotti, Charles F. Dewhurst, Lia W. Liefting, Lastus Kuniata, Titus Kakul, Putative Vectors of a Phytoplasma Associated with Coconut (Cocos nucifera) in Madang Province, Papua New Guinea, International Journal of Agriculture and Forestry, Vol. 4 No. 5, 2014, pp. 365-372. doi: 10.5923/j.ijaf.20140405.04.
Article Outline
1. Introduction
- Lethal-yellowing (LY) in various forms, affects a large number of palms throughout the world. Danyo (2011) [1] indicated that at least 40 species of palms may be affected although Cocos nucifera (coconut) has been the main species investigated due to its economic importance in some countries. Symptoms of LY in coconut include, but are not restricted to yellowing of leaflets, premature nut fall, flower necrosis and spear rot. The agents responsible for LY-type diseases in palms are mainly phloem-inhabiting phytoplasmas of different genetic origin [2], [3], [4].Coconuts are cultivated extensively in coastal areas of Papua New Guinea (PNG) and are an integral part of the lifestyle as well as being a cash crop for rural populations. Coconut palms displaying LY-like symptoms including leaflet yellowing and necrosis, frond collapse and premature nut fall and their subsequent death in coconut groves and old plantations were first reported in several villages in the Bogia District of Madang Province, PNG, in 2008 [5]. The colloquial name “Bogia Coconut Syndrome” (BCS) was given to describe this disorder. A phytoplasma related to but distinct from the coconut lethal yellowing group (16SrIV) was detected in a few samples obtained from symptomatic coconuts in 2008 [6]. Further sampling of coconut palms and banana plants from Bogia revealed a phytoplasma by nested-PCR. The phytoplasma from coconut palms was identified as that reported by Kelly et al. 2011, [6] (C. Pilotti, unpublished). Davis et al. 2012, [7] subsequently identified a phytoplasma from banana plants with similar yellowing symptoms from neighbouring Provinces of Morobe and East Sepik, referring to it as Banana wilt associated phytoplasma (BWAP). The 16S rRNA gene sequences from the phytoplasma associated with BCS and BWAP are identical. Since coconuts and bananas are important crops in PNG, this finding brought forth the need to implement control and containment programmes. Besides restriction of plant movement from Madang Province, screening of insect populations was necessary to confirm that the agent causing symptoms in banana and coconut was also present in insect fauna in the same locality. As phytoplasmas are phloem-inhabiting bacteria-like organisms that are transmitted by phloem-feeding insects [8], [9], our study focused on species within the Order Hemiptera which includes plant and leaf hoppers many of which are sap feeders with mouthparts adapted to piercing and sucking sap from leaves and soft tissues of plants. Because of their mode of feeding, potential vectors of BWAP would most likely be found among species within this group [9], [10]. Auchenorrhyncha (Hemiptera) have long been associated with diseases in coconut [11] but according to Weintraub 2007, [9] vectors of phytoplasmas have only been confirmed from among the families Cicadellidae, Cixiidae, Delphacidae, Derbidae, Flatidae and Psyllidae. For example, Myndus spp. (Cixiidae) are plant hoppers responsible for diseases in coconut in Vanuatu and Christmas palm (Veitchia sp.) in Florida [12], [13], [14]. In the search for vectors of lethal yellowing-like diseases (LD) in Tanzania Mpunami et al. 2000, [15], screened a large number of insects collected from LD infected coconut areas and positive products were found in a few individuals of Diostrombus mkurangi (Derbidae) and Meenoplus spp. (Meenoplidae).Despite the apparent widespread occurrence of phytoplasmas in coconuts in Africa, Asia and the Caribbean [2], [16], many of the vectors of LY type coconut diseases have not been identified. The vector for the long known Cape St Paul wilt disease (CSPWD) of coconuts in Ghana remains elusive although two species, a Derbidae (Diostrombus sp.) and a Cixiidae, Myndodus adiopodoumeensis (Myndus adiopodoumeensis) were found to contain the CSPWD phytoplasma but transmission trials were inconclusive (1), [17].Recently, Dollet et al. 2011, (4) detected the LY Group 16SrXXII phytoplasma in pentatomid bugs (Platacantha lutea) collected from areas in Mozambique where coconuts were displaying symptoms associated with lethal yellowing type syndrome (LYTS). This study was initiated as a prerequisite to more detailed research on the biology of BWAP, which will ultimately include identifying the vector (s), understanding the biology and transmission and determining the host plant range of this phytoplasma.The search for potential insect vectors of BWAP was undertaken in Madang Province where reports of BCS originated and where earlier sampling of coconuts revealed the presence of BWAP (C. Pilotti, unpublished).
2. Materials and Methods
2.1. Field Collections
- Insects were collected from Furan (majority of collections), Omuru and Vidaro in Madang Province (Fig. 1). Collections were made during April 2012 (end of the rainy season) and from August to October 2012 (lower rainfall period). Target insect taxa were Hemiptera (especially Cicadellidae, Derbidae, Delphacidae, Flatidae and Lophopidae). Collections were made by either “tubing” single insects directly from the plants or by using nets to sweep specimens from the fronds or branches of larger trees. All insects were placed individually into 95% ethanol after collection. Representative specimens of insects collected were retained by PNG Oil Palm Research Association (PNGOPRA) at Dami, West New Britain Province and at the National Agricultural Insect Collection (NAIC) at Kilakila, Port Moresby, PNG as reference materials.
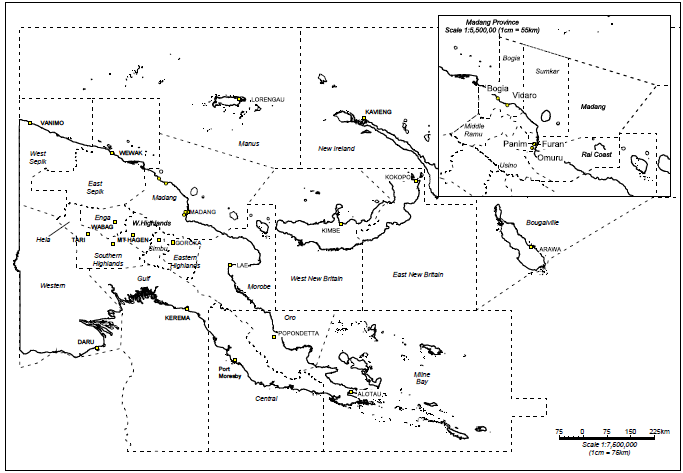 | Figure 1. Map of Papua New Guinea showing the Madang Province and inset: the main locations of insect collection sites in Madang Province |
2.2. Sample Preparation
- Individual insects were removed from alcohol and placed on paper towels for a minimum of 1h to evaporate the alcohol. DNA was extracted from whole insects or insect heads using the Qiagen DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. DNA quality was checked by electrophoresis to ensure adequate amounts of high molecular weight DNA in the extracts.
2.3. PCR Analysis
- PCR was carried out using phytoplasma-specific (16S rRNA gene) primers and TopTaq™ Master Mix Kit (Qiagen) according to the manufacturer’s instructions. The reaction volume of 25μl contained 1 × master mix with 5µM primers. In the first round PCR, the primers used were R16mF2/R16mR1 [18] with 1µl undiluted template DNA. Nested-PCR was carried out on 1µl undiluted or 1/20 diluted templates using primer pair R16F2n/R16R2 [18]. Conditions used were as also described in Gunderson & Lee, 1996 [18] using a Techne gradient thermal cycler. For first round PCR: 2min. at 94℃ then 35 cycles of 1min. at 94℃, annealing for 2min. at 60℃, primer extension 3min. at 72℃ with a final step of 10min. at 72℃. Nested-PCR conditions were as for first-round PCR but with an annealing temperature of 55℃. Positive (LY phytoplasma) and no template controls were included with each batch of samples analysed. After amplification, 10μl of the reaction mixture was run on 1.2% agarose gels and stained with ethidium bromide for viewing and photography.The 1,250bp R16F2n/R16R2 products from 30 insects were sequenced directly using the same primers at EcoGene®, Auckland, New Zealand. Sequence analysis was performed in Geneious version 5.5.6 created by Biomatters (http://www.geneious.com/). Forward and reverse sequence reads were trimmed to remove regions of low quality and were then assembled resulting in fragments ranging from 943bp to 1,146bp. The consensus sequences were searched against the GenBank database for homologous sequences using BLASTn [19] network service available at the National Centre for Biotechnology Information (Bethesda, MD, USA). The sequence from one Zophiuma pupillata (Hemiptera: Lophopidae) specimen has been submitted to Genbank (Accession # KJ826504).
3. Results
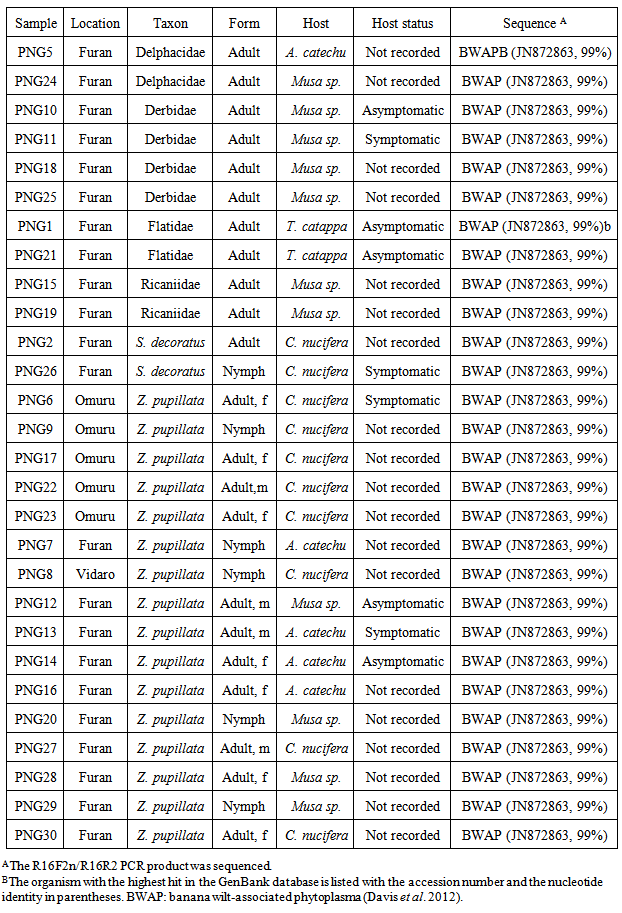
- Samples were deemed positive for phytoplasma if the nested-PCR produced a detectable band of the same size as the positive control (1,250bp). Samples which produced an amplicon in the first-round PCR but not in the nested-PCR were considered to be negative with the cause likely due to other bacteria being amplified (Harrison et al. 2002(3).Of the 302 insect samples analysed by nested-PCR, 96 (32%) were positive for phytoplasma (Table 1). Z. pupillata (Fig.2B) was the most frequently collected insect, and phytoplasma was detected in 57/121 (47%) of samples tested. Phytoplasmas were detected in nymphs as well as adults of both sexes. The majority of Z. pupillata were collected from coconut and betel nut hosts, although a small number were also collected from bananas (Table 1). Specimens from all three hosts tested positive for phytoplasma as did one insect collected from Pandanus sp. Sequence analysis of 16 Z. pupillata confirmed that the phytoplasma detected by nested-PCR was BWAP (Table 2).
|
|
4. Discussion
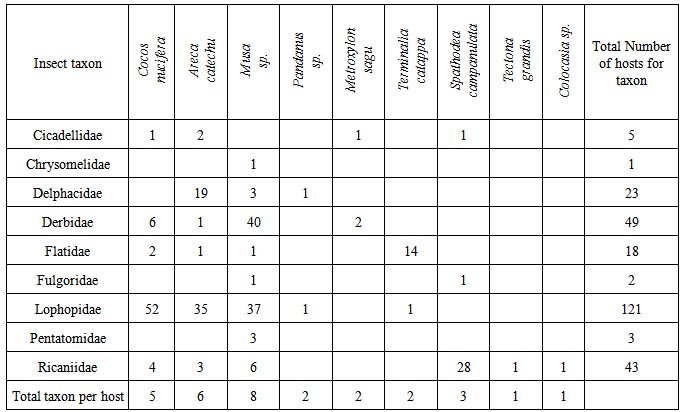
- Hemiptera were target taxa in this study as they are known sap-feeders and vectors of diseases on palms in other countries [8], [10]. A number of different insect species representing Hemiptera were found at the sampling sites in Madang Province. BWAP was detected and confirmed by sequencing in specimens from the hemipteran families Delphacidae, Derbidae, Flatidae, Lophopidae, Pentatomidae and Ricaniidae and Tettigonidae (Orthoptera). The most abundant insect in the locality was Z. pupillata (Hemiptera: Lophopidae). This species appears to be polyphagous as it was found on coconut, betel nut and banana plants in significant numbers with a few specimens collected from T. catappa. The high numbers found on palms and banana plants and its scarcity on other hosts indicate that Z. pupillata is primarily associated with monocotyledons. The common occurrence of Z. pupillata on coconut palms in and the observation of egg masses on coconut frond bases suggest that the species may complete its breeding cycle on coconut as does Z. butawengi (formerly Z. lobulata) [20]. Little is known about this insect and the male of this species was only recently described [21]. A closely related species, Z. butawengi is the vector for Finschhafen Disorder of coconut in PNG while Painella simondsi has been associated with a yellowing disorder of coconuts in Solomon Islands however, phytoplasmas have not been implicated in these disorders [22]. Z. pupillata is a potential vector for transmission of BWAP in coconut because it was widespread on coconuts at the sampling sites. Insects were collected from both symptomatic and asymptomatic plants at Furan, Omuru and Vidaro. A high proportion of the Z. pupillata from Omuru were found to contain BWAP. The significance of the results from Omuru is not clear. There may be highly susceptible coconut germplasm in the area or an abundance of alternative hosts at Omuru which could contribute to a localised epidemic. The large number of these hoppers observed at Omuru would also increase the probability of acquisition or transmission of the phytoplasma to and from host plants and this may be the reason for the high detection rate of BWAP. Clearly, more detailed studies are required in this area. Specimens of Delphacidae were collected from betel nut palms and banana plants at Furan. Species within this family are known carriers and vectors of phytoplasmas and are primarily associated with Poaceae worldwide [8], [10], [16]. Several species of Delphacidae are responsible for phytoplasma diseases in rice and sugar cane in Asia, Cuba and PNG [16].We also suggest that species within this family are potential vectors and may contribute to the spread of BWAP amongst other monocotyledons as they were found on betel nut, banana and Pandanus sp. hosts. However, because of their low abundance they are not considered to be primary vectors of BCS in coconut but they could contribute to persistence of BWAP in other hosts such as grasses. A species of Derbidae tentatively identified as Proustia sp. was collected almost exclusively from banana plants around Furan Village. Many of the specimens were obtained from asymptomatic banana plants and it was not possible to correlate PCR-positive assays with symptoms in banana. All other collections of Derbidae were from palms (coconut, betel nut and sago), and this suggests that there is a capacity for this insect to transmit BWAP from coconut to banana or vice versa. Notably, Proustia moesta is recorded as a vector of Coconut root wilt disease and Sugarcane grassy shoot disease in SE Asia (16). In addition, 16SrIV phytoplasmas have been detected in Cedusa sp. (Derbidae) in Jamaica [23]. Brown et al. 2006 (23) found that almost half of the small number of Derbidae analysed carried different strains of the 16SrIV group phytoplasma although they were all closely related to the strains in Florida (97-98% similarity). This has interesting implications for future work on BCS although in this study, BWAP was not found to vary among the different host insects. This may be due to the uniformity of the organism in the host plant species from which the insects fed. More extensive collections of insect fauna in the area may prove otherwise. Insufficient information is available about the feeding behaviour of the Derbidae recorded in this study to make any conclusions as to their role in the transmission of phytoplasmas amongst host plants. However, from the evidence in other tropical countries [16], [23], and because of their abundance on banana and betel nut hosts in the study area, it is proposed that these insects are likely vectors of BWAP and should be targeted in future transmission studies. Davis et al. 2012, (7) found different phytoplasmas in banana plants from other Provinces but it is noted that in this study, all insects extracted and tested from banana carried only a single organism.The few specimens testing positive for phytoplasma from families such as Cicadellidae, Flatidae and Pentatomidae suggest that these species are not primary vectors of BWAP and may be chance carriers. The low numbers of these taxa found and collected from the study site reinforce this view but this could be a seasonal effect, and it is important to carry out surveys throughout the year to obtain representative collections of insect fauna. It is noteworthy that some species of Cicadellidae and Pentatomidae complete their life cycle on palms (10), and Pentatomidae eggs were collected from coconut palm leaflets during this study. The16SrXXII group phytoplasma responsible for lethal yellowing type syndrome (LYTS) in coconuts in Mozambique was identified in P. lutea (Pentatomidae) feeding on symptomatic palms and it was hypothesised that this species is a vector of the causal agent (4). Insects within this family should therefore continue to be investigated in future studies.Two Ricaniidae (Hemiptera) specimens that contained BWAP were collected from banana plants, one symptomatic and the other asymptomatic. The small number of PCR-positive assays (4/45, 9%) obtained for this insect species indicates a low capacity for transmission of phytoplasmas as they were quite abundant in the area but more frequently collected from the dicotyledon tree Spathodea campanulata (African Tulip) although a small number were also found on coconut and betel nut palms. This may be the first record of phytoplasmas in species of Ricaniidae.Positive PCR assays were obtained for S. decoratus collected from coconut and betel nut, and BWAP was confirmed by sequencing. This is probably the first report of Tettigoniidae being tested for phytoplasmas as these insects are not usually target taxa in studies on phloem-inhabiting bacteria. Since they are large grasshoppers that chew on the leaflets of palms, the presence of BWAP is most likely a result of contamination of the mandible from feeding on infected plant tissue. Indeed, one of the specimens was collected from a symptomatic coconut palm.In many cases the status of the host plants from which insect collections were made was not recorded hence, strong associations between phytoplasmas in insect and plant hosts could not be ascertained in this study. Some coconut, betel nut and banana plants sampled from within the study area contained BWAP (C. Pilotti, unpublished).There may be more than one vector of the BWA phytoplasmas at Furan, Omuru and Vidaro, and this possibility presents some challenges with regard to control of BCS. Weintraub 2007, (9) indicated that in many cases, vector host range is the major factor that limits spread of phytoplasma diseases rather than the behavior of the insect vectors themselves. Even given this scenario, it will be the polyphagous vectors with large mobile populations that will be the most difficult to manage in our quest to contain BCS in Madang Province.
5. Conclusions
- This study has identified species within the hemipteran families Delphacidae, Derbidae, Flatidae and Lophopidae as carriers of BWAP, and we propose some species within Derbidae and Lophopidae as putative vectors of BCS in Madang Province. Further research is required to clarify the role of these insects in the transmission of BWAP, determine their distribution, and identify appropriate control measures.
ACKNOWLEDGEMENTS
- This survey was funded by ACIAR under a Small Research (SRA) grant to PNG OPRA. We thank Dr Richard Markham for his continued and enthusiastic support for this research.We are grateful for the assistance of staff from PNGOPRA, RAI and CCI with insect collections.The LY phytoplasma positive control used in this study for the nested-PCR in PNG was kindly supplied by Dr Nigel Harrison, University of Florida.This paper is published with authority of the Director of Research, PNGOPRA.
References
| [1] | G. Danyo, 2011. Review of scientific research into Cape St. Paul Wilt Disease (CSPWD) of coconut in Ghana, African Journal of Agricultural Research, 6, 45, 67-4578. |
| [2] | A. Mpunami, P. Tymon, P. Jones and Dickinson 1999. Genetic diversity in the coconut lethal yellowing disease phytoplasmas of East Africa, Plant Pathology, 48, 109-114. |
| [3] | N. A. Harrison, M. Womack and M. L. Carpio, 2002. Detection and characterisation of a lethal yellowing (16SrIV) group phytoplasma in Canary Island date palms, Plant disease, 86, 676-681. |
| [4] | M. Dollet, F. Macome, A. Vaz and S. Fabre, 2011. Phytoplasmas identical to coconut lethal yellowing phytoplasmas from Zambesia (Mozambique) found in a pentatomide bug in Cabo Delgado Province, Bulletin of Insectology, 64 (Supplement), 139-140. |
| [5] | A. B. Kembu, W. Yasinge, D. Tenakanai, and N. Bangulass, 2009. Delimiting Survey of an Infectious Coconut Syndrome in Bogia District of Madang Province: a Preliminary Report, March 2009. PNG Cocoa Coconut Institute. 43pp. |
| [6] | P. L. Kelly, R. Reeder, P. Kokoa, Y. Arocha, T. Nixon and A. Fox, 2011. First report of a phytoplasma identified in coconut palms (Cocos nucifera) with lethal yellowing like symptoms in Papua New Guinea, New Disease Reports, 23, 9. |
| [7] | R. I. Davis, P. Kokoa, L. M. Jones, J. Mackie, F. E. Constable, B. C. Rodoni, T. G. Gunua, J. B. Rossel ,2012. A new wilt disease of banana plants associated with phytoplasmas in Papua New Guinea, Australasian Plant Disease Notes, 7, 91–97. |
| [8] | M. R. Wilson and P. G. Weintraub, 2007. An introduction to Auchenorrhyncha phytoplasma vectors. Bulletin of Insectology, 60, 177–178. |
| [9] | P. G. Weintraub, 2007. Insect vectors of phytoplasmas and their control – an update, Bulletin of Insectology, 60, 169-173. |
| [10] | M. R. Wilson, 1997. Leafhopper and planthopper faunas (Homoptera: Auchenorrhyncha) of coconut palms in lethal yellowing areas, Proc. of the International Workshop on Lethal Yellowing-like Diseases of Coconut. Elmina, Ghana. Eds: SJ Eden-Green, F.Oferi, 1997, Chatham UK. Natural Resources Institute. |
| [11] | F. Bonnot, G. Danyo, R. Phillipe, S. Dery, A. Ransford, 2009. Preliminary results on epidemiology of Coconut Lethal Yellowing in Ghana, OCL, 16, 116-122. |
| [12] | J. F. Julia, 1982. Myndus taffini (Homoptera: Cixiidae): Vector of foliar decay of coconut in Vanuatu, Oleagineux, 40, 19-27. |
| [13] | F. W. Howard and F. W Mead. 1980. A survey of Auchenorrhynca (Insecta: Homoptera) associated with palms in Southern Florida, Tropical Agriculture Trinidad, 57, 145-153. |
| [14] | F. W. Howard, R. C. Norris and D. L. Thomas, 1983. Evidence of transmission of palm lethal yellowing agent by a planthopper Myndus crudus (Homoptera: Cixiidae). Tropical Agriculture Trinidad, 60, 168-171. |
| [15] | Mpunami A., Tymon P., Jones P., Dickinson M. J. 2000. Identification of potential vectors of the coconut lethal disease phytoplasma. Plant Pathology, 49: 355-361. |
| [16] | P. G. Weintraub and L. Beanland, 2006. Insect vectors of phytoplasmas, Annual Review of Entomology, 51, 91-111. |
| [17] | R. Phillipe, J. P. Nkansah, S. Fabre, R. Quaicoe, F. Pilet and M. Dollet, 2007. Search for the vector of Cape St. Paul Wilt (coconut lethal yellowing) in Ghana, Bulletin of Insectology, 60, 179-180. |
| [18] | D. E. Gunderson and I-M. Lee 1996. Ultrasensitive detection of phytoplasmas by nested-PCR assays. Phytopatholgia Mediterranea, 35, 144–151. |
| [19] | S. F. Altschul, T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, D. J. Lipman, 1997. Gapped BLAST and PSI BLAST: a new generation protein database search programs, Nucleic Acids Research, 25, 3389-3402. |
| [20] | Smith, E.S.C. 1980. Zophiuma lobulata Ghauri (Homopetera Lophopidae) and its relation to Finschhafen disorder in Papua New Guinea, Papua New Guinea Agricultural Journal, 37, 37-45. |
| [21] | C. W. Gitau, M. J. Fletcher, A. Mitchell, C. F. Dewhurst and G. M. Gurr, 2009. Review of the planthopper genus Zophiuma Fennah (Hemiptera: Fulgoromorpha: Lophopidae) with first description of the male of Zophiuma pupillata Stålaen. Australian Journal of Entomology, 50, 86–92. |
| [22] | M. R. Wilson, 1986. The Auchenorrhyncha (Homoptera) associated with palms, Proc. Workshop on Leafhoppers and Planthoppers of Economic Importance. Eds. Wilson M. R. and Nault L. R. CIE London, 1987. Pp.327-342. |
| [23] | S. E. Brown, B. O. Been, W. A. McLaughlin, 2006. Detection and variability of the lethal yellowing group (16SrIV) phytoplasmas in the Cedusa sp. (Hemiptera: Auchenorrhyncha: Derbidae) in Jamaica, Annals of Applied Biology, 149, 53-62. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML