-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(5): 351-358
doi:10.5923/j.ijaf.20140405.02
Commercial Broilers Exposed to Aflatoxin B1: Efficacy of a Commercial Mycotoxin Binder on Internal Organ Weights, Biochemical Traits and Mortality
Hedayati M.1, M. Manafi1, M. Yari1, S. V. Mousavipour2
1Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
2Private Section Veterinary Clinician, Qom, Iran
Correspondence to: Hedayati M., Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to determine the binding capacity of a commercial mycotoxin binder (BINDER) for aflatoxin B1 (AF) and the efficacy of the binder to reduce the adverse effects of AF on broilers fed AF. Two hundred and Forty 1-d-old unsexed broilers (Ross 308) were maintained in chick batteries and allowed ad libitum access to feed and water. A completely randomized design was used with 3 replicate pens of 20 chicks assigned to each of 5 dietary treatments from hatch to 42 d. Dietary treatments included the following: A) control (CON), with no binder or AF, B) AF supplemented at the rate of 0.6ppm, C) 0.2% binder, and D) 0.6ppm AF supplemented with 0.2% binder. On d 42, 8 chicks from each treatment were killed by cervical dislocation and samples of visceral organ weight, cholesterol, HDL, LDL and mortality percentage were recorded. Results showed that weights of Thymus and Kidney had not been affected and Spleen, Liver and Pancreas and cholesterol, HDL and LDL were affected by incorporation of AF in the diet. The addition of binder to the affected parameters could restore some of these adverse effects significantly. Incorporation of only binder had reached the mortality into zero.
Keywords: Aflatoxin B1, Mycotoxin binder, Visceral Organs, Cholesterol, LDL, HDL, Broilers
Cite this paper: Hedayati M., M. Manafi, M. Yari, S. V. Mousavipour, Commercial Broilers Exposed to Aflatoxin B1: Efficacy of a Commercial Mycotoxin Binder on Internal Organ Weights, Biochemical Traits and Mortality, International Journal of Agriculture and Forestry, Vol. 4 No. 5, 2014, pp. 351-358. doi: 10.5923/j.ijaf.20140405.02.
Article Outline
1. Introduction
- In 2012, approximately 82.9 million metric tons of broilers were produced. Nutrition plays as the most important issue in rearing poultry. As per the FAO, 25% of World’s cereals are contaminated with mycotoxin. Mycotoxins are an increasingly discussed issue and among them, aflatoxin is the most dangerous and poisonous one in poultry. The toxigenic strains of Aspergillus flavus, Aspergillus parasiticus, and Aspergillus nomius produce a group of secondary metabolites such as aflatoxins, which are known to be potent mutagenic, carcinogenic, teratogenic, hepatotoxic, immunosuppressive and also inhibit several metabolic systems [1]. Aflatoxins are fungal metabolites found as contaminants in a wide range of food and agricultural products. Aflatoxin B1, the most commonly occurring aflatoxin, is a potent mutagen and hepatocarcinogen to a wide range of animal species [2]. Chronic exposure to aflatoxins may not only significantly alter productivity and animal farming trends, but may also impose a risk to the consumer from direct exposure to aflatoxin-contaminated food commodities [3]. Formations of these toxins are linked to fungal growth and the environment in which the grains/cereals are stored (especially relative humidity and temperature). Fungal growth and subsequent mycotoxin production in stored grains can be inhibited by physical methods (aeration, cooling, modified atmospheres, etc.) or by fungi stats of which the propionic, acetic and sorbic acids are the most commonly used [4]. These toxins have been incriminated as the cause of high mortality in poultry and livestock and some cases of death in human being [5]. Thus foods contaminated with these toxigenic fungi and presence of aflatoxin is a major concern, which has received worldwide attention due to their deleterious effects on human and animal health as well as their importance in international food trade [6].Aflatoxin B1 is widely believed to result in mal-absorption syndrome regarding macro nutrients and also in reduced activity of digestive enzymes [7]. However, many reports contrary to this notion are available. For instance, Nelson et al. [8] did not find any effect of AFB1 (natural contamination of corn with A. flavus) on dry matter (DM) and amino acid digestibility and energy utilization in chicken. Applegate et al. [9] did not find any effect of 0.6, 1.2 and 2.5 ppm AFB1 in diet on digestibility of DM and nitrogen (N) per hen/day. At 0.6 and 1.2 mg AFB1/kg diet, the apparent metabolizable energy (AME) was however found to be reduced in their study. Regarding the activity of pancreatic enzymes, Matur et al. [10] found higher amylase and chymotrypsin activity, while lower lipase activity after exposure of Ross 308 female birds to 0.1 mg AFB1/kg diet (at 427 to 457 days age). The activity of trypsin in pancreas was not affected by AFB1 treatment. These results, except for reduction in lipase activity, are supported by earlier work of Richardson and Hamilton [11] on layers. These authors reported that 4 ppm AFB1 in diet increases the activity of pancreatic chymotrypsin, amylase and lipase. Pancreatic trypsin was not affected by AFB1 in their study and the noted changes in the pancreatic secretions were also not reflected in the lipid content of the feces. Contrary to these two reports, Osborne and Hamilton [12] noted lower activity of pancreatic amylase, trypsin, lipase, RNAse, and DNAse when broilers were exposed to 1.25 and 2.5 mg AFB1/kg diet.The use of numerous plant extracts, spices and their constituents may provide an alternative way to prevent fungal growth and aflatoxin formation [13]. It is believed that the extracts of certain spices and herbs of medicinal importance exhibit antifungal property. These natural antifungal agents can be potentially exploited in controlling the growth of fungi and consequently inhibiting aflatoxin formation [14]. The addition of antioxidants is a method of increasing the shelf-life, especially of lipids and lipid containing foods. Synthetic antioxidants, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene, have restricted use in foods as these synthetic antioxidants are suspected to be carcinogenic [15]. Therefore, the importance for search of natural antioxidants especially of plant origin has greatly increased in recent years [16].Curcumin (diferuloylmethane), a polyphenol, is an active principle of the perennial herb Curcuma longa (commonly known as turmeric). The yellow-pigmented fraction of turmeric contains Curcuminoids, which are chemically related to its principal ingredient, curcumin. The major Curcuminoids present in turmeric are curcumin I (77%), demethoxycurcumin (curcumin II) (17%),bisdemethoxycurcumin (curcumin III) (3%) and the recently identified cyclocurcumin. Throughout the Orient, it has traditionally been used to good therapeutic effect, particularly as an anti-inflammatory [17] and many of its therapeutic effects have been confirmed by modern scientific research. Such effects include antioxidant [18], antiinflammatory [19-21], anticarcinogenic and antimicrobial [22], hepatoprotective [23], thrombosuppressive [24], cardiovascular (i.e., as protection against myocardial infarction) [24], hypoglycemic [25] and antiarthritic (i.e., as protection against rheumatoid arthritis) [26]. In another study, it has been reported that turmeric and curcumin, inhibited mutation frequency by more than 80 percent. Dietary administration of turmeric (0.05 per cent), garlic (0.25 per cent), curcumin and ellagic acid (0.005 per cent each) to rats significantly reduced the number of gamma glutamyl transpeptidase-positive foci induced by AFB1.One of turmeric's components is curcumin, a type of phytochemical known as a polyphenol. Research findings suggest that phytochemicals, which are the chemicals found in plants, appear to help prevent disease. As the bioactive component of turmeric, curcumin is readily absorbed for use by the body. The addition of products containing minerals from clay products to AFB1 contaminated diets has been shown to greatly reduce the bioavailability of aflatoxin in the gastrointestinal tract [5]. The liver is the target organ of AFB1 in broilers and is characterized by a severe hepatic enlargement and fatty infiltration [27]. The liver and kidney are the main organs involved in the detoxification of AFB1, and also the place which most residues accumulate [28]. The AFB1 is primarily biotransformated in the liver by cytochrome P-450 associated enzymes, which generate hydroxylated metabolites [2]. Diatomaceous earth refers to a naturally-formed sedimentary mineral coming from the remains of what were once oceanic unicellular shells and algae known as diatoms. Diatoms, the ocean's "spiny honeycombs," are over 30 million years old, and were formed when these microscopic algae-like plants died and remained compounded in the earth's surface as skeletal remains [29]. These organisms, much like a mollusc emits lime-carbonate, had the ability to emit silica. Scientists refer to these clay-like, chalky remains as diatomite [30]. Clays are natural adsorbents chemically made of silicates or aluminosilicates. They include a large range of products such as hydrated sodium calcium aluminosilicates (HSCAS), phyllosilicates (of which montmorillonite or magnesium hydrated HSCAS is one of the major compounds in this group), bentonite and zeolite (the latter two are clays of volcanic origin). Silica is also known as Diatomaceous Earth, made up of 84% Silicon Dioxide (Silica). Bentonite clay carries a uniquely strong negative ionic charge which causes it to “magnetically” attract any substance with a positive ionic charge (i.e., bacteria, toxins, metals, etc.). These substances are both adsorbed (sticking to the outside like Velcro) and absorbed (drawn inside) by the clay molecules. The clay`s immediate action upon the body is directly on the digestive channel. This involves the clay actually binding with the toxic substances and removing them from the body with the stool. It performs this job with every kind of toxin, including those from the environment, such as heavy metals, and those that occur naturally as by-products of the body`s own health processes, such as metabolic toxins. The clay and the adsorbed toxins are both eliminated together; this keeps the toxins from being reabsorbed into the bloodstream [28].Due to limited forage supplies, more poor quality feeds, such as those high in nitrates, lignin, ash, and mycotoxins will be fed. Feeding extra minerals can help mitigate the negative effects of feeding poor quality forages. Clearly much of the pioneering work with mycotoxin binders was done with silicates and specifically with the HSCAS material. These binders have the property of adsorbing organic substances either on their external surfaces or within their inter-laminar spaces, by the interaction with/or substitution of the exchanged cations within these spaces. Therefore, mycotoxins can be adsorbed into this porous structure and be trapped by elementary, electric charges. However, clay and zeolitic minerals, which comprise a broad family of diverse aluminosilicates, are not produced equally and thus; do not possess the same physical properties [13]. Eralsan et al. [30] reported a moderate increase in the albumin: globulin ratio of broilers by addition of 0.3 per cent hydrated sodium bentonite in aflatoxin mixed feed of broilers. They also reported that histopathological finding in liver sections of broiler fed aflatoxin plus hydrated sodium bentonite indicated a non-protective effect of this adsorbent. Due to their montomorillonite content, bentonites swell and form thixotropic gels, as result of their ion exchange capabilities, they are widely used as mycotoxin sequestering agent [31]. Eralson et al. [32] reported the effectiveness of sodium bentonite in reliving the damages due to the presence of aflatoxins (1ppm) in 45 day old broiler chickens.The aims of the current study were to determine the activity of the commercial mycotoxin binder containing curcuminoids, minerals and enzymes and also to evaluate the protective effects of the binder on internal organ weights, biochemical traits and mortality of broilers fed with Aflatoxin B1.
2. Materials and Methods
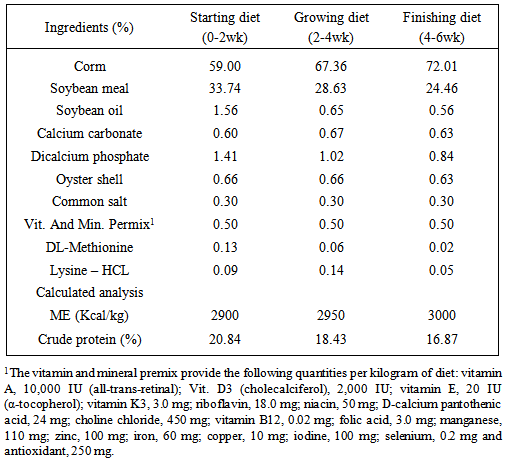
- This experiment was planned and carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with objective of evaluating the internal organ weights, biochemical traits and mortality of broilers fed with aflatoxin B1 and a mycotoxin binder. Experimental design, housing, management and test diet240 day-old unsexed Ross 308 strain of broiler chicks were wing banded, weighed and randomly spread in a completely randomized experimental design with four treatments and three replications of twenty chicks in each. Each replicate group of chicks was housed in an independent pen, conventional deep litter house. Chicks in all the replicates were kept up to six weeks of age under uniform standard conditions. Brooding was done till three weeks of age. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4, the lighting schedule was 24 hour. At days 14-42 the dark time was gradually increased to 4 hour. Diets were prepared to meet the nutrient requirements of commercial broilers during the starter (0-2wks), grower (2-4wks) and finisher (4-6 wks) periods. The composition of diets was adopted from NRC, [33] and is presented in Table 1. The basal diet was formulated using commonly available feed ingredients which were screened for AF prior to the formulation of diets and it is found to be around 280ppb in normal feed. The Aflatoxin B1 was procured from Sigma Aldrich, USA and diluted to reach to the required level of administration. The experimental diets were prepared by adding required quantity of aflatoxin to arrive at the level of 600ppb of AFB1. Diets were prepared without addition of aflatoxin and binder as Control (group 1); 600 ppb Aflatoxin B1 (group 2); 0.2% of binder (group 3) and 600ppb Aflatoxin B1 + 0.2% of binder (group 4). Niltox, the mycotoxin binder used in this study is a unique composition of minerals (extra purified clay containing diatomaceous earth mineral), antioxidants (Curcuminoids extracted from Turmeric) and enzymes (Epoxidase and Esterase), a property product of Zeus Biotech Limited, Mysore, India. It is claimed that incorporation of this product in poultry diets would effectively prevent DNA adduct formation and cellular damages in the biological systems through degrading peroxides, amides and lacto rings in non-polar toxins such as aflatoxins. This study was undertaken to evaluate the efficacy of a mycotoxin binder for counteracting AFB1 in experimentally contaminated broiler breeder diets.
|
3. Results and Discussion
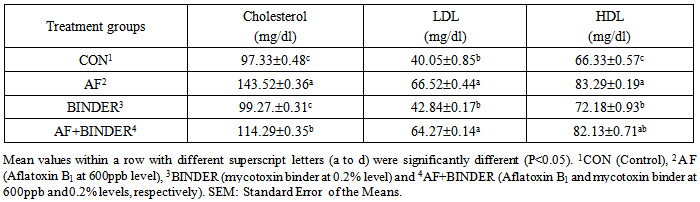
- The data on visceral organ weights of broilers fed aflatoxin, toxin binder and their combinations at 42 day of age are shown in Table 2. Results showed that Thymus weight has not been affected by any dietary treatments. The weight of Spleen has been decreased significantly (P<0.05) in AF fed group and while adding binder to the AF diet, these reduction could significantly (P<0.05) restored. The binder alone fed treatment also has shown a significantly (P<0.05) lesser in Spleen weight of broilers. The bursa of Fabricius weight has increased in AF treatment and by addition of toxin binder, the weight is decreased. The binder alone fed group is also significantly (P<0.05) decreased, when compared with control group. The Liver weight has also been increased in AF fed group, In AF+BINDER group, the liver weight is decreased to even below the control group level. The Kidney weight has not been affected in different treatments. The Pancreas weight had been decreased significantly (P<0.05) in AF group and by adding binder to AF group remained unchanged.
|
|
|
4. Conclusions
- The purpose of this study was to introduce the activity of curcuminoids, minerals and enzymes, as a mycotoxin binder in broilers on internal organ weights, biochemical traits and mortality of broilers fed with Aflatoxin B1. Results obtained from this study concludes that besides the adverse and negative effects of aflatoxin B1 in the diet on visceral organ weights, some biochemical parameters and mortality of broilers with a commercial mycotoxin binder can rule its beneficial effects to some extent. However, there is insufficiency in the marks of its beneficial effects in nutrient digestibility and gut function of broilers.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML