-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(4): 338-342
doi:10.5923/j.ijaf.20140404.11
The Changes in Phenolic and Flavonoids Compound Related to the Harvest Times and Enzyme Activity during Stages of Leaves Development in Juglans Regia L
Heidar Ali Malmir
Associate Professor, Laboratory of Plant Cell physiology, Department of Biology, BU-Ali Sina University, Hamedan, Iran
Correspondence to: Heidar Ali Malmir, Associate Professor, Laboratory of Plant Cell physiology, Department of Biology, BU-Ali Sina University, Hamedan, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
This study was conducted to investigate the activities of polyphenol oxidase (PPO) and Phenylalanine ammonialyase (PAL) total phenolic acid contents (TPC), total flavonoids content (TFC), radical scavenging capacity (RSC) and water contents (WC) during development stages of walnut leaves. The first plant samples were obtained 20 days after foliar started and the others were obtained each 20 day intervals up to the end of leaf growth. The results of multiple regression showed that increase in PPO activity over the seven harvests had a negative effect on the TPC and WC (p< 0.05), whereas PPO activity had a slightly positive effect on the TFC and RSC during the stages of leaf development (p< 0.05). The PPO activity and RSC gradually increased (p<0.05), whereas the PAL activity decreased and the concentrations of TFC increased steadily from the first sample until early September (p< 0.05). This was in contrast with the increase in concentration of TPC during early leaf growth and after budding to decline in mid June. The WC have a stronger effect on synthesize alterations in TFC and TPC of the leaves compared to the PAL and PPO activities. These results suggest that the synthesize alterations has been related to the enzymes activities and were linked with WC, leaf development and overall decreased cell wall plasticity.
Keywords: Walnut, Enzyme activity, Synthesize alterations, Leaves developments
Cite this paper: Heidar Ali Malmir, The Changes in Phenolic and Flavonoids Compound Related to the Harvest Times and Enzyme Activity during Stages of Leaves Development in Juglans Regia L, International Journal of Agriculture and Forestry, Vol. 4 No. 4, 2014, pp. 338-342. doi: 10.5923/j.ijaf.20140404.11.
Article Outline
1. Introduction
- The walnut tree is frequently cultivated for its edible seed in the Tuyserkan areas of Iran. It is also the dominant species in latitudinal and altitudinal in the Sarabi River. It is usually a polychromic clonally tree and the leading producer of this crop in the Tuyserkan. The Persian or common walnut is its best-known member constituting an important species in Iran and commercially cultivated in the Tuyserkan [10]. Walnuts have one of the highest contents of RSC among all trees. Phenolic compounds are antioxidant metabolites, which occur in abundance in all plant material [1]. The polyphenols compounds, especially the flavonoids, consist mainly of anthocyanidins, flavonols, catechins and participate in cell protection against the harmful action of reactive oxygen species ROS [3]. Mainly oxygen free radicals are produced in response to environmental stresses such as salinity, drought, high light intensity or mineral nutrient deficiency [7]. During development of leaves walnut, polyphenolic compounds are gradually modified. Reactions among anthocyanins, flavan-3-ol, proanthocyanidins and other compounds, such as glyoxylic acid, pyruvic acid and acetaldehyde, and also between flavonols themselves, have been observed. These reactions are responsible for the appearance of new polyphenol [1, 12, 13]. The different between phenolic compounds were grouped according to the degree of polymerization and changed in time extraction. Low molecular weight phenols monomers catechin and epicatechin (TFC) there are highly reactive compounds which are involved in condensation and polymerization processes by them. High molecular weight phenols (TPC) the galloyl derivatives did show important changes during development and extraction was increased only when sufficient time was allowed for maceration [1, 4].The PPO has been found in higher plants and is responsible for enzymatic browning of raw fruits and vegetables. PAL is regarded as the enzyme which controls the flow of phenylalanine into phenolic compounds [12, 13]. There are not enough studies on the polyphenol composition of the Persian walnut fruits and leaves. This experiment was conducted to evaluate PAL and PPO activities and TPC, TFC, WC and RSC during the leaf development, in order to identify physiological and biochemical characteristics of this compound and related enzymes in the leaves of walnut trees.
2. Materials and Methods
- The study area was the Tuyserkan including surrounding which is located in Hamedan province, Iran (36°46′N, 48°34′E). The climate in the study area is variation and fairly cold and altitude from sea level was 2000 meter. The temperature in daytime was about 24°C and at night 10°C. Soil pH measured 6.9-7.2, (CaCl2 1:2 v: v). the experimental design used was a randomized complete block design and the trees were 20 years old. Four replicates for each harvest were prepared to give a total of 28 trees. The first plant samples were obtained 20 days after foliar started and the others were obtained each 20 day intervals up to the end of leaf growth (from the 20th of May until the 20th of September 2008). The WC was estimated from the difference between FW leaf and DW leaves.
3. Determination of TPC and TFC
- The TPC of the acetone extracts were measured using the Folin–Ciocalteu reagent adapted from the method of Chimi et al. (1991), [3]. This is a sensitive and quantitative method, independent of the degree of polymerization. Diluted samples (250 µL-1) were added to deionized water (1.8 mL-1) followed by Folin-Ciocalteu reagent (250 µL-1). After 3 min sodium carbonate (500 µL-1, 35% w/v) was added, followed by deionized water (1.4 mL-1). Total phenolics acid (samples) + alkaline + FC reagent + heat = blue colored product and the absorbance was recorded at 755 nm. Results were expressed as milligrams of Gallic acid equivalents per gram of FW. TFC were analysed by the method of Solar et al. (2006), [15]. For the optimization of TFC from walnut leaves, 2mL of sample at 1 mg/mL-1 in methanol contained in a PTFE screw capped vial, the equal volume of the 2% AlCl3 solution (2g in 100 mL-1 methanol) was added. The mixture was vigorously shaken and absorbance was read at 415 nm after 1 h of incubation at 20°C. Results are expressed in qucertin equivalents (mg QE/g of FW extract).
4. Determination of Radical Scavenging Capacity (RSC)
- The RSC of leaves was tested in methanolic extracts by the free 2.2 diphenyl-1-picrylhydrazyl (DPPH) method Awika et al. (2003), [2]. The reaction mixture was incubated in the dark for 15 min and the absorbance was recorded at 517 nm. %IP = [(Ac - As)/Ac] x 100Where Ac and As are the absorbencies of the control and of the test sample after 15 min, respectively.
5. The PPO (EC; 1.14.18.1) and PAL (EC; 4.3.1.5) Activity Assay
- PPO activity was assayed spectrophotometrically as described by Ortega-Garcia et al. (2008) [12], using DL-3, 4-dihydroxyphenylalanine (DL-DOPA) as a substrate. The reaction mixture containing 1.3 ml of 0.03 M potassium phosphate (pH 6.5) buffer and 1.2 ml L-DOPA was heated to 30°C for 2 min and finally 0.5 ml of the enzyme extract was added to the cuvette. Changes in the absorbance at 475 nm were measured for 3 min using a Schimadzu UV-120-01 spectrophotometer. Enzyme activity was expressed as “AA475/min/g fresh weight”.Activity of PAL was measured according to the method of Ortega-Garcıa et al. (2009), [13], with slight modifications. 1 ml of enzyme extract was incubated with 2 ml of borate buffer (50 mM, pH=8.8) and 1 ml of L-phenylalanine (20mM) for 60 min at 37°C. The reaction was stopped with 1ml of 1M HCl. The assay mixture was extracted with 3ml of toluene by vortexing for 30 sec. The absorbance of toluene phase containing trans-cininamic acid was measured at 290 nm. Enzyme activity was expressed as nmol trans-cinnamic acid released h-1g-1 FW.
6. Statistical Analysis
- A first-order multiple regression covariance structure was used to test the effect of HT (as a fixed factor) on the concentrations of compounds and enzymes activities.
7. Results and Discussion
- Polyphenolic deposition in plants leaves are one of the mechanisms which allowed the plants adapted to a terrestrial habitat. Their synthesis results from a partially known long chain of reactions which represent one of the most expensive biosynthetic processes in plants in terms of energy demand [12, 13]. In the present study, water content was high in young leaves but decreased during development to approximately 67% for most of the summer (Fig. 1a). The content of TPC compounds were highest at the mid of June (18.6 mg/g FW) and this increase subsequently fall with advent of season (9.4 mgg-1 FW; Fig. 1b). In early leaves development, walnut leaf TPC is the main sink for phenylalanine and can be highly influenced by water content (Fig. 2b). The TFC contents of the leaves studied here are shown in (Fig. 1b and 3b). The lowest level of TFC was detected in the early harvest (17.8 mgg-1 FW), whereas the content was gradually increased during the leaf development (32.3 mgg-1 of FW). Among the differences observed, the increase of TFC in the four and five harvests is notable. These results are in agreement with those [5, 8, 15].the relationship between leaf FW and accumulation of TPC and TFC (Fig. 3b, 3c) for TPC, the transformation activity in leaf walnut is much higher than the rate of synthesis, and for TFC the rate of synthesis, in leaf walnut is much less than the transformation activity, indicating that synthesis of TFC continued through most of the summer at rates exceeding the rates of leaf growth in terms of fresh weight accumulation. Changes in the relative proportion of TPC and TFC are currently observed in this experiment, depending on the time and water contents. The levels of TPC and TFC in the leaves are the result of the balance between biosynthesis and catabolism. (Fig. 1b, 2b).The results from this study support the hypothesis of this study is that a direct relationship exists between PAL, PPO and the concentrations of TFC and TPC in walnut leaves were investigated (Fig. 1c, 2a). From Figures it has been noted that PPO activity is positively correlated with TFC (Table 1; R2 = 0.98) and negatively correlated with total TPC (R2 = -0.85) in leaves. When the TPC decreases, the PPO activity in leaves increases (Fig. 1c). The results from this experiments showed that an oxidation of phenolic by PPO was gradually increased during the leaves development and we have suggested that this may be due to the transformation of soluble phenolic into insoluble cell-wall-bound constituents polyphenol [8, 12, 15]. From a physiological point of view, these results suggest that the early growth stage could be characterized by the maximum TPC synthesis and maximum growth period of leaves. After this stage, the leaves must reduce its growth and prepare itself to the development stage (Fig. 1b). In fact, it could be postulated that during maturation stage of leaves, the leaves decreased TPC and provide to prepare itself to the lignifications process in order to slow down its growth [9]. Indeed, many studies reported that lignin is a polymer synthesized from TPC compounds of phenylpropane type [14, 15]. This process may be directly related to the formation of cell walls and argued that capacity was directly related to the activity PPO enzyme [12]. The TPC compounds play a key role in toughness of leaf. A negative correlation between reduced fibber bound condensed tannin and the toughness in leaf of sorghum was found [8]. The results of many study indicated that difference between trees with low and high concentration of TPC and TFC could be explained at least in part by phenological differences among the trees [11]. One group of phenylpropanoids are TFC, the most important water-soluble pigments in plants. It is even possible that the function of the TPC may be contacting and binding of phenolic acid with the proteins and production protein bound condensed tannin of the cell wall [6]. The PAL activity is showing a continuous downward trend during leaf maturation from Juan up to December. (Fig. 2c, 1c; R2 = -0.81). The results from other experiments showed that, the first reaction in the phenylpropanoid pathway is catalysed by PAL converting L-phenylalanine to trance-cinnamic acid [9, 13]. One of the major leaves changes is RSC associated with the enzyme PPO and PAL activities (Fig. 2c). Similar results were also obtained in other studies [7]. The correlation data role of TFC as RSC and in growth and development of leaf is clearly shown (Fig. 3a). The negative correlation during growth seasons of spring and summer is observed between TPC and RSC, which showed that TPC consumed up in growth and development of the plant and thus RSC increases with time as the seasons going on (Fig. 2c, 3a; Table 1, p<0.05). Thus our results seem to indicate that RSC of polyphenol extracts could be attributed to TFC (Fig. 3a).
- It is believed that the TFC increases with the number of available OH groups in the B ring of the TFC. The number of available OH groups in the B ring of the TFC more than TPC and increased during leaf development [11]. The decrease in PAL activity might be controlled by either RSC potential of TFC or activation by stress (Fig. 2c). A comparison of WC of the leaves and the levels of RSC of walnut at seven stages of development leaf shows that there are significant differences in changes of WC and RSC was found (Fig. 3c; Table 1, P<0.05). It seems that WC could affect significantly on the RSC and composition of TFC and TPC [7].
8. Conclusions
- Our results show that the relationship between PPO and PAL activities and accumulation of TPC and TFC. For TPC, in the early growth stage the transformation activity in leaf walnut is much higher than the rate of synthesis and after this stage, the leaves decreased TPC and increase TFC, indicating that synthesis of TFC continued through most of the summer at rates exceeding the rates of leaf growth. Changes in the relative proportion RSC are currently observed in this experiment, depending on the harvest time, TFC and WC. The walnut leaves can be used as an easily accessible source of natural RSC.
Abbreviations
- PPO, polyphenol oxidase; PAL, Phenylalanine ammonialyase; TPC, total phenolic acid content; TFC, total flavonoids content; RSC, radical scavenging capacity; WC, water contents; HT, harvesting time; FW, fresh weight; dry weight, DW.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML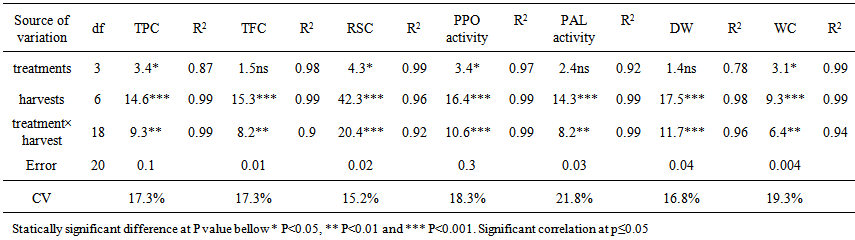
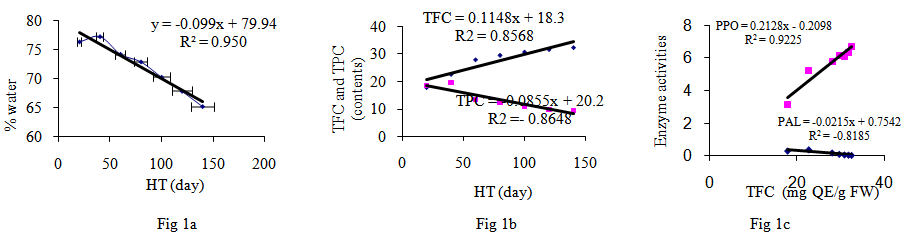
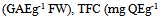 FW) and HT during leaves development in walnut. Fig 1c: The relationship between TFC and enzymes PAL (µmol cinnamic
FW) and HT during leaves development in walnut. Fig 1c: The relationship between TFC and enzymes PAL (µmol cinnamic 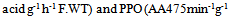 FW) activities during leaves development
FW) activities during leaves development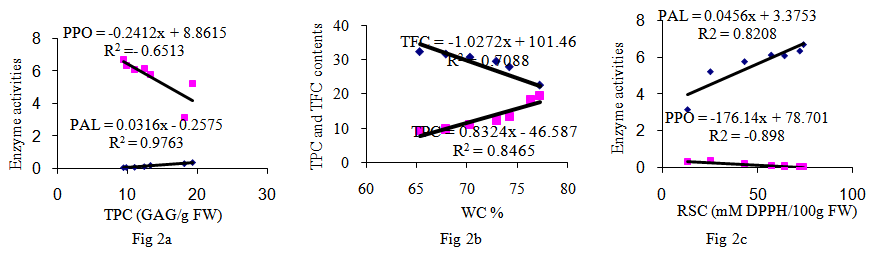
 FW) and PPO (AA475/min/g FW) activities during leaves development. Fig 2b: The relationship between TPC mg (GAG)
FW) and PPO (AA475/min/g FW) activities during leaves development. Fig 2b: The relationship between TPC mg (GAG)  F.W and TFC (mg
F.W and TFC (mg  FW) and WC during leaves development. Fig 2c: The relationship between RSC activities and Enzyme PAL (µmol cinnamic acid
FW) and WC during leaves development. Fig 2c: The relationship between RSC activities and Enzyme PAL (µmol cinnamic acid 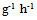 F.WT) and PPO
F.WT) and PPO 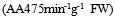 activities during leaves development
activities during leaves development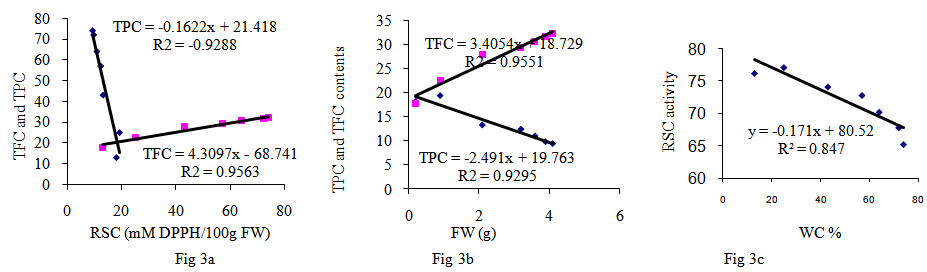
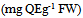 during the development of leaves. Fig 3b: The relationship between TPC mg
during the development of leaves. Fig 3b: The relationship between TPC mg 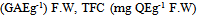 and FW during leaves development. Fig 3c: The relationship between RSC (mM
and FW during leaves development. Fig 3c: The relationship between RSC (mM 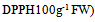 activities and water contents during leaves development
activities and water contents during leaves development