-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(4): 286-292
doi:10.5923/j.ijaf.20140404.04
The Efficacy of Thyme Essence on Internal Organ Weights, Biochemical Traits and Mortality of Broilers Fed Aflatoxin B1
Manafi M., M. Hedayati, M. Yari
Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran
Correspondence to: Manafi M., Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
A total of 240 day old chicks were used in a completely randomized design to investigate the effects of Thyme essence, in aflatoxin B1 contaminated diets on the internal organ weights, biochemical traits and mortality of broilers. Twelve pen replicates with 20 chicks in each pen were assigned into each of the 4 dietary treatments having 3 replicates comprising: 1) basal diet containing; 2) basal diet + 600ppb aflatoxin; 3) basal diet + 500ppb Thyme essence; and 4) basal diet + 600ppb aflatoxin + 500ppm Thyme essence. The addition of aflatoxin to diets reduced spleen weight and increased the liver weight and inclusion of Thyme essence could only restore the increased liver weight. Aflatoxin increased the cholesterol, LDL and HDL levels in the broilers blood and Thyme essence cold partially reduce these increased levels. In conclusion, results of the present study indicated that the Thyme essence is a partially useful effective for reducing the signs of aflatoxin B1 toxicities in growing broilers.
Keywords: Aflatoxin B1, Thyme Essence, Body Weight, Feed Consumption, FCR, Antibody titers, Broilers
Cite this paper: Manafi M., M. Hedayati, M. Yari, The Efficacy of Thyme Essence on Internal Organ Weights, Biochemical Traits and Mortality of Broilers Fed Aflatoxin B1, International Journal of Agriculture and Forestry, Vol. 4 No. 4, 2014, pp. 286-292. doi: 10.5923/j.ijaf.20140404.04.
Article Outline
1. Introduction
- Aflatoxins, secondary metabolites of various Aspergillus spp., commonly pollute a wide variety of tropical and subtropical food/feed stuffs. These mycotoxins are known to have strong hepatotoxic and carcinogenic properties [1]. Chemically, aflatoxins are furanocoumarin mixtures and include B1, B2, G1, G2, M1, and M2. These mycotoxins contaminate a wide variety of agricultural commodities including oilseed meals, dried fruits, spices, and cereals [2]. Among the various types of aflatoxins, aflatoxin B1 (AFB1) is most frequently encountered and it is also considered to have higher toxicity than other aflatoxins. A study revealed the impact of low concentrations of aflatoxin, deoxynivalenol (Vomitoxin) or fumonisins in diets on growing pigs and poultry [3]. In this study, they used simple linear regression to summarize different trials from the literature and estimated the relationship between mycotoxin level and for example, growth rate. The researchers estimated that for each additional part per million (ppm) of aflatoxin in the feed, the growth rate of pigs is depressed by 16%. For instance, a level of 0.3ppm aflatoxin in feed would result in a 5% reduction in daily weight gain when compared to a non-contaminated feed. For deoxynivalenol, the reduction in daily weight gain was estimated at about 8% for each additional ppm. Similarly, it was calculated that growth performance of pigs is reduced by 0.4% for each additional ppm of fumonisins in the diet. Decreased water and feed intake, weight loss, dullness, stunting, ruffled feathers, poor appearance and paleness, trembling, ataxia, lameness, paralysis of the legs and wings gasping, prostration and death are most seen in experimental and natural outbreak of aflatoxicosis in broilers [4]. The most characteristic gross lesions appeared in the livers which were enlarged, pale yellow to grayish brown and had a prominent reticular pattern. The liver, spleen and kidney will increase in size, whereas the bursa of Fabricius and thymus will be decreased [5]. Lethal aflatoxicosis can cause either dark red or yellow discoloration of the liver due to congestion or fat accumulation, respectively. At chronicity livers became shrunken, firm and nodular and gall bladder was distended. The kidneys of affected birds appeared enlarged and congested and the spleen will be enlarged and mottled in appearance [6]. Histopathology of the liver revealed congestion of hepatic sinusoids, fecal hemorrhages, centrilobular fatty cytoplasmic vacuolation and necrosis, biliary hyperplasia and nodular lymphoid infiltration. In the kidney, the epithelial cells of many tubules were vacuolated. Another study reported severe degeneration of hepatocytes, dilation of central veins, bile duct proliferation and lymphocytic depletion in lymphoid organs in field outbreaks of aflatoxicosis in broilers [7]. Several biochemical parameters are affected by aflatoxin exposure [8]. Aflatoxin decreases total serum proteins, alpha, beta and gamma globulins, with IgG being more sensitive than IgM [6]. Total serum proteins contents are depressed due to reduced values of alpha and beta globulins and albumen, while gamma globulins are affected more variably. Serum lipoproteins, cholesterol, triglycerides, uric acid and calcium are also decreased [7]. The activity of serum or plasma enzymes has been widely used as a measure of aflatoxin activity in chickens. Increased activities of sorbitol dehydrogenase, glutamic dehydrogenase, lactate dehydrogenase, alkaline phosphatase, acid phosphatase, aspartate aminotransferase and alanine aminotransferase were reported in aflatoxin fed chickens [4]. The rise in the levels of serum enzymes measured was interpreted as a consequence of hepatocyte degeneration and subsequent leakage of enzymes [4]. Aflatoxin treated birds showed decreased fractional excretion of phosphate, total plasma calcium concentration, decreased total plasma proteins, plasma 25-hydroxyl vitamin D and plasma 1, 25-dihydroxy vitamin D. Essential oils are complex compounds, and their chemical composition and concentrations of various compounds are variable [9]. Essential oils basically consist of two classes of compounds, the terpene and phenylpropene, depending on the number of 5-carbon building blocks. The exact antimicrobial mechanism of essential oils is poorly understood. However, it has been suggested that their lipophilic property [10] and chemical structure [11] can play a role. It was suggested that terpenoid and phenylpropanoid can penetrate the membranes of the bacteria and reach the inner part of the cell because of their lipophilicity [12]. Moreover, structural properties, such as the presence of the functional groups [11] and aromaticity are also responsible for the antibacterial activity of essential oils. Various plant extracts, especially essential oils, have been studied for their antimicrobial abilities. Most of researches done in this area have been performed in vitro, but there have been few studies with live poultry flocks. A recent study involving live birds showed that blends of the primary components of the essential oils could be used to control Clostridium perfringens, the bacterium that causes necrotic enteritis in broilers [13]. Ground thyme has been shown to inhibit the growth of S. typhimurium when added to media .The essential oil of the thyme has been shown to inhibit the growth of the E. coli in media [14]. Aromatic plants and essential oil extracted from these plants have been used as alternatives to antibiotics. For this reason, these plants are becoming more important due to their antimicrobial effects and the stimulating effect on animal digestive system [15]. Cinnamon extract inhibits Helicobacter pylori at the concentration range of common antibiotics, its antimicrobial properties are mainly related to its cinnamaldehyde (the organic compound that gives cinnamon its flavor and odor) content, followed by eugenol and carvacrol substances. The effect of ground thyme and cinnamon on the performance of broilers was studied by Al-Kassie [16], who found their effect on the live weight gain and the improvement of the health of poultry, in addition to other performance traits, feed conversion ratio and feed intake. The aim of this study was to find the effect of oil extract from thyme and cinnamon as natural growth promoting substances in broiler chicks. Thyme (Thymus vulgaris) is a species of flowering plant in the mint family Lamiaceae, with the main components of Phenols, thymol (40%) and carvacrol (15%). This herb also used customarily for several medicinal purposes: respiratory disease, antimicrobial, anti-nociceptive and etc. [17]. Thymol and carvacrol are the main antibacterial active substances, so this plant can be used instead of commercial antibiotics. A group of scientists reported beneficial value of thyme in poultry industry [15]. Thyme is known in Iran as Shirazian thyme and is a popular medicinal plant in whole country. The active components of thyme are thymol and carvacrol, which are known for their antioxidant and antibacterial properties [18]. The in vivo reports on the effect of thyme on blood parameters and immunity are very limited, whereas the benefits of probiotics on the immune system are becoming more widely accepted. The purpose of current study was to investigate the effect of substitution of ground thyme as a phytobiotic and a probiotic. In another study [19], it has been reported the occurrence of a significantly (P<0.01) higher carcass yield in broiler chicks fed with the probiotics on the 2nd, 4th and 6th week of age. Moreover, the effect of a mixture of herbal essential oils on growth and internal organ weight of broilers and concluded that there were no significant effects for the dietary treatment on body weight of the broilers at 21 and 42 days.
2. Materials and Methods
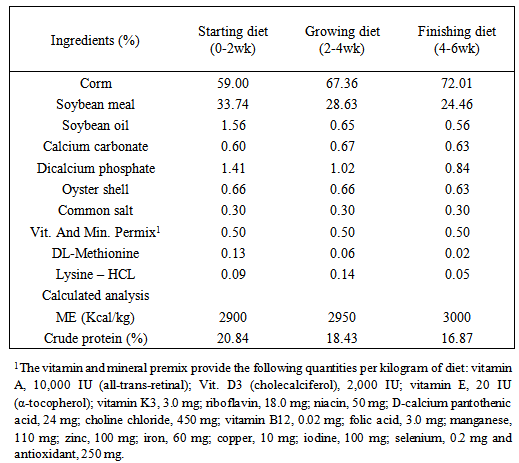
- This experiment was planned and carried out in the Department of Animal Science, Faculty of Agricultural Sciences, Malayer University, Malayer, Iran with objective of evaluating the performance and immune response of broilers fed with aflatoxin B1 and Thyme essence. Experimental design, housing, management and test diet240 day-old unsexed Ross 308 strain of broiler chicks were wing banded, weighed and randomly spread in a completely randomized experimental design with four treatments and three replications of twenty chicks in each. Each replicate group of chicks was housed in an independent pen, conventional deep litter house. Chicks in all the replicates were kept up to six weeks of age under uniform standard conditions. Brooding was done till three weeks of age. Each pen was fitted with an automatic bell type drinker and a hanging tubular feeder. Chicks were provided ad libitum feed and water throughout the study. Feeding of test diets commenced at first day of age and continued till the termination of experiment at six weeks of age. The temperature was maintained at 30±1°C in the first week and reduced by 2.5°C per week to 21°C. From day one until day 4, the lighting schedule was 24 hour. At days 14-42 the dark time was gradually increased to 4 hour. Diets were prepared to meet the nutrient requirements of commercial broilers during the starter (0-2wks), grower (2-4wks) and finisher (4-6 wks) periods. The composition of diets was adopted from NRC [20] and is presented in Table 1. The basal diet was formulated using commonly available feed ingredients which were screened for AF prior to the formulation of diets. The Aflatoxin B1 was procured from Sigma Aldrich, USA and diluted to reach to the required level of administration. The experimental diets were prepared by adding required quantity of aflatoxin to arrive at the levels of 0 and 600ppb of AFB1. Diets were prepared without addition of aflatoxin and Thyme essence as Control (group 1); 600 ppb Aflatoxin B1 (group 2); 500ppm of Thyme essence (group 3) and 600ppb Aflatoxin B1 + 500 ppm of Thyme essence (group 4). The ethanolic extraction of Thyme was prepared as per the instruction given below:
|
3. Results and Discussion
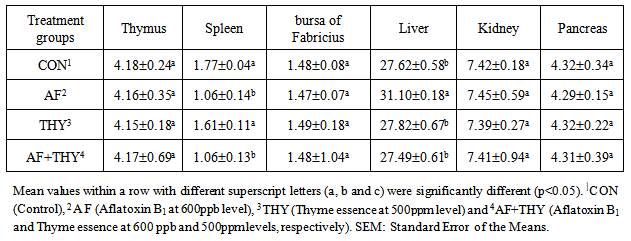
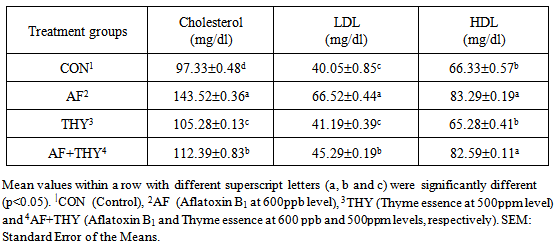
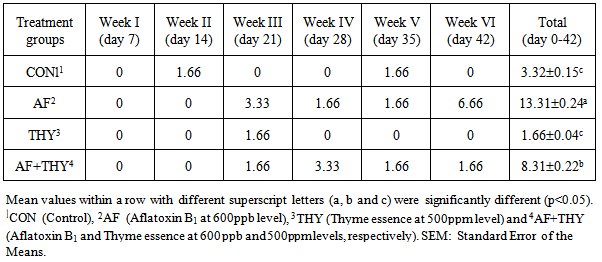
- The effects of aflatoxin and Thyme essence on Visceral Organ weights (g/kg live weight): The results of dietary treatments on organ weights of broilers fed AF and Thyme and their combinations on broilers at 42 days of age are shown in Table 2. Results showed that the thymus weights have no statistical differences among all treatment groups and ranged from 4.15 to 4.18 g/kg BW. Spleen weights in both AF fed group (Groups 2 and 4) were decreased significantly (P<0.05) which shows the adverse effects of AF on the spleen weight. The THY data on this organ was as good as control group and showed no significant change. In case of bursa of Fabricius, no significant changes were noticed among all treatment groups and could be said that all feed additives had no effects of the weight of this internal organ. Liver weights have been influenced by the treatments and in AF group, a significant (P<0.05) increase in the weight of liver has been found. The liver weight, in THY group was as equal as CON and no significant changes were noticed. In AF+THY fed group, the liver weight is decreased to that of control and by comparing this group with AF alone fed group, the weight is significantly decreased (P<0.05). In case of Kidney and pancreas weights, no impacts of any of treatment groups have been found and these data remained non-significant for all treatments.The effects of aflatoxin and Thyme essence on blood biochemical parameters (mg/dL): The results of dietary treatments on blood biochemical parameters of broilers fed AF and Thyme and their combinations on broilers at 42 days of age are shown in Table 3. Results showed that inclusion of AF in the diet (treatment 2) has increased the cholesterol level of broilers significantly (P<0.05), compared with control group and in THY group, this parameter is differed significantly (P<0.05) with control group. By comparing the F with AF+THY fed group, the data showed that Thyme essence has decreased the cholesterol level below that of AF group significantly (P<0.05) and could be said that Thyme essence has a positive effect on the cholesterol level during aflatoxicosis. The LDL content of AF fed group was increased significantly (P<0.05) and restored to some extent by addition of Thyme essence (treatment 4) significantly (P<0.05). In Thy alone fed group, the LDL level has been remained no-n significant, when compared with control group. Almost the same trend is found in HDL (mg/dL) content of broilers blood at 42 days stating that in AF fed group, the HDL values is increased significantly (P<0.05), when compared with control group. In THY group, the HDL value is remained as equal as control group, but surprisingly, the Thyme essence added to AF (AF+THY) could not restore the increased value of HDL due to aflatoxicosis. The effects of aflatoxin and Thyme essence on Mortality (%): The results of dietary treatments on weekly mortality of broilers fed AF and Thyme and their combinations on broilers at 42 days of age are shown in Table 4. Results showed that during first week, there was no dead bird in all treatment groups. During the second week, only control group has shown a 1.66% of mortality and other groups remained 0. The rate of mortality for the second week (15-21 days) was found to be 0 in CON, 3.33 in AF fed group, 1.66 in THY and 1.66% in AF+THY group. During the week IV, the data on mortality rate (%) of different treatments were 0, 1.66, 0 and 3.33 in CON, AF, THY and AF+THY fed groups, respectively. At week V, the mortality found in CON was 1.66, AF 1.66, THY 0 and AF+THY 1.66. In the last week of experiment, the mortality for control and Thyme essence fed groups remained zero and in AF fed group was found 6.66 and in AF+THY fed group was found to be 1.66. The overall data on percentage mortality in different groups found to be 3.32, 13.31, 3.32 and 8.31 in CON, AF, THY and AF+THY treatment groups, respectively. This shows that the increased percentage of mortality could be controlled by inclusion of Thyme essence in the diet.
|
|
|
4. Conclusions
- Based on the founded data in this trial and available reports, it could be concluded that the addition of aflatoxin in broiler diet can have a negative impact on the Visceral Organ weights, blood biochemical parameters and mortality of broilers and Thyme essence can be used as natural non-antibiotic feed additive in broiler nutrition. Nevertheless, there is scarcity in the evidence of its beneficial effects in nutrient digestibility and gut function of broilers fed with aflatoxin and Thyme essence.
ACKNOWLEDGEMENTS
- This study was funded by Directorate of Research, Malayer University, Malayer, Iran.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML